Answer to Q1
Let’s label the circuit as follows:
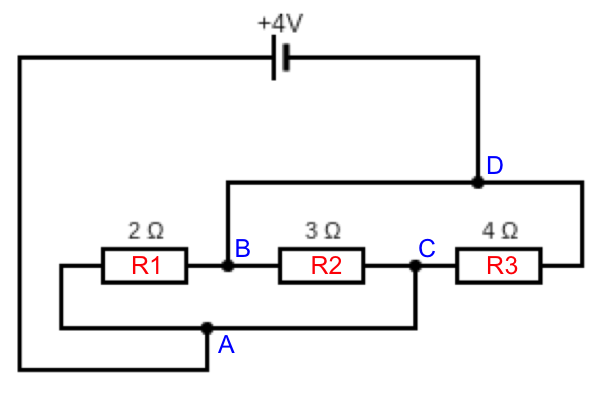
The above circuit can be redrawn as:
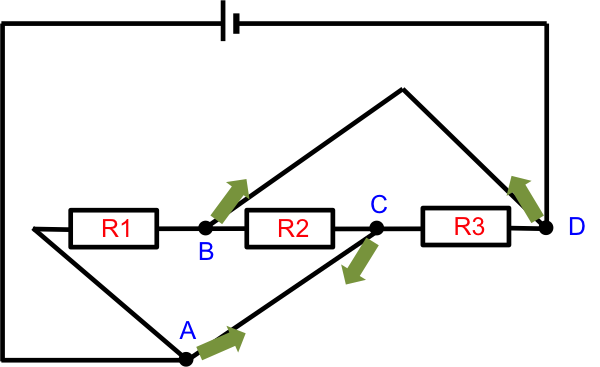
Since junctions A and C have the same potential, we can simplify the diagram by combining them into a single junction (imagine stretching and pulling points A and C towards each other and merging them into one junction). Similarly, junctions B and D have the same potential and can be combined into a single junction. The resultant circuit looks like this:
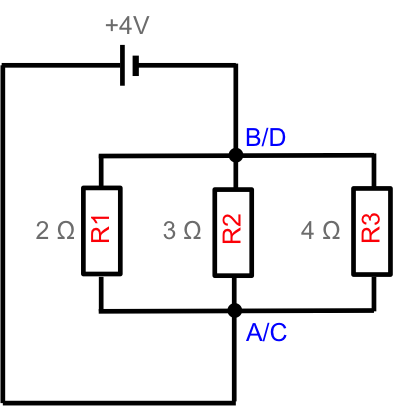
It is a simple circuit with three parallel resistors of effective resistance . The current flowing in the circuit is therefore
.