Titration, or titrimetry, is an analytical technique for determining the concentration of a chemical substance called an analyte by reacting it with a solution of known concentration.
There are many different titration methods, including acid-base titration, redox titration, complexometric titration, gravimetry titration, potentiometric titration, coulometric titration, thermometric titration and radiometric titration.
This article focuses on acid-base titration and redox titration. In an acid-base titration (e.g., HCl and NaOH), if the analyte is an acid, the reacting solution is the base, which is added in consecutively small amounts (using the apparatus shown in the diagram below) to the other. The analyte can be either in the burette (the dispensing device) or in the conical flask.
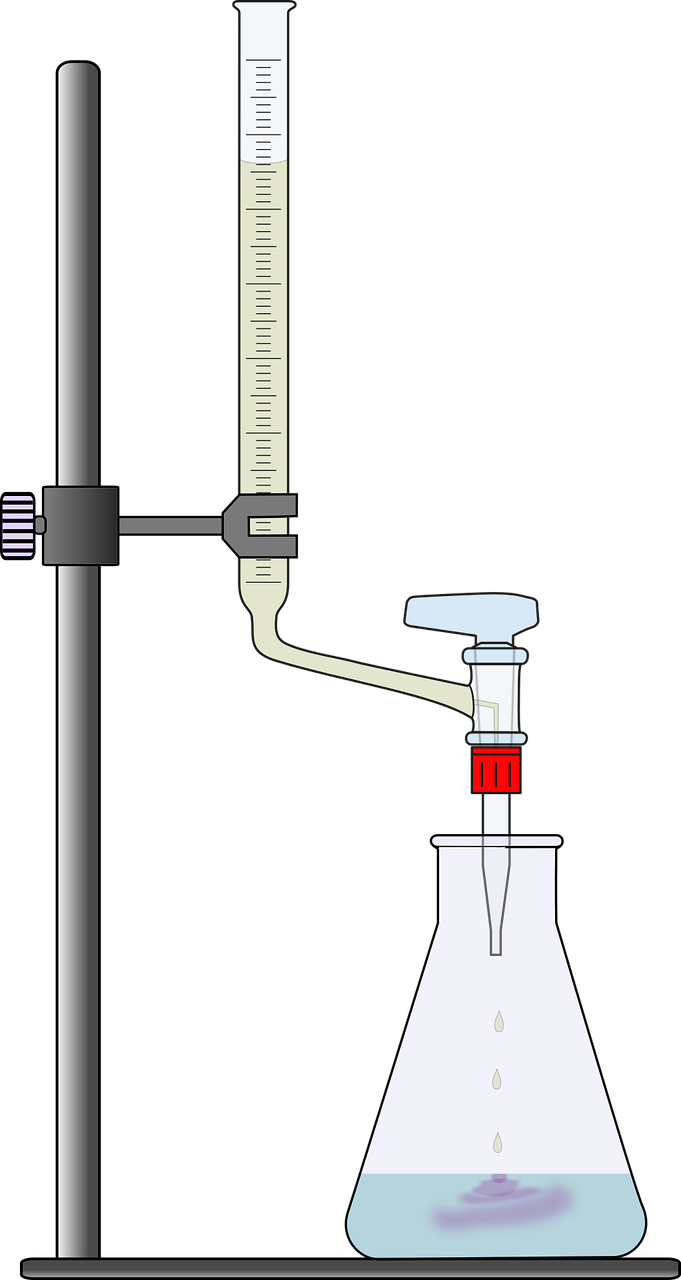
During the preparation stage of the titration, the burette is filled with an acid (or base) through a glass funnel, which is inserted into the burette’s top opening. A pipette (a chemical dropper) is used to introduce the base (or acid) to the conical flask. A few drops of an auxillary reagent called an indicator (a colour changing compound) is also added before start of the reaction. The reaction begins when small amounts of the reagent is added from the burette to the substance in the conical flask and is complete when a significant change of colour of the solution in the flask is observed. The concentration of the analyte is then calculated via stoichiometric analysis by noting the volume of solution added from the burette to the flask.
For example, a solution in a flask containing 25.0 cm3 of an unknown concentration of NaOH with a few drops of methyl orange (indicator) appears red at the beginning of the titration. The solution remains red as 0.1 M HCl is gradually added from the burette. Just before reaching the equivalence point, the solution turns orange, which signals to the person conducting the experiment that the next couple of drops of HCl will turn the solution yellow (the end point), where the titration is complete. The concentration of NaOH is calculated with the volume of HCl used (e.g. 12.5 cm3) and by referring to the neutralisation equation:
According to the stoichiometry of the above reaction, moles of HCl react with
moles of NaOH. Therefore,
In a redox titration (e.g. Fe2+ and MnO4– with phosphoric (V) acid), the same procedures apply but the indicator may not be required as either the analyte or the solution of known concentration may have the ability to change colour to pinpoint the completion of the reaction.
In the above titration, MnO4– is added from the burette to Fe2+. Therefore, the solution in the flask remains colourless until the end point, where the solution turns pale pink.

Question
Why is an acid added to a permanganate-to-iron titration and why is H3PO4 the preferred acid as compared to HCl?
Answer
The oxidising strength of MnO4– varies with the pH of its solution. At low and high pH, MnO4– is reduced by Fe2+ to Mn2+ and MnO2 respectively. Since MnO2 is a brown precipitate, it is impossible to observe the end-point if the titration is carried out in an alkaline medium. Furthermore, both Fe2+ and Fe3+ form coloured and colourless complexes with Cl– and PO43- respectively. As it is easier to observe the pale pink end-point in a colourless solution than a yellow solution, H3PO4 is preferred.