Joseph Gay-Lussac, a French scientist, is often credited with the discovery of the pressure-temperature law in the early 1800s even though Guillaume Amontons, another French scientist had conducted a similar experiment earlier on with the same conclusion. Gay-Lussac’s experimental apparatus involves immersing a gas-filled, fixed-volume bulb-like copper container in a temperature controlled water bath. The container is connected to a pressure gauge, which measures the pressure of the gas in the container at various temperatures (see diagram below).
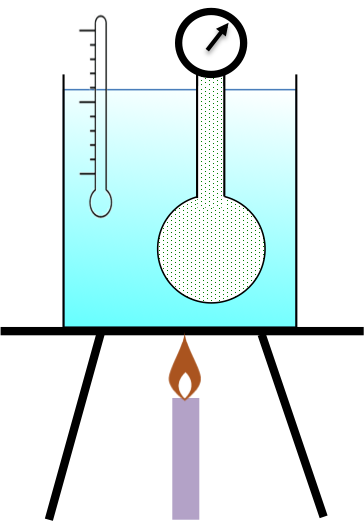
Both Gay-Lussac and Amontons found that the relationship between the pressure of a gas and its temperature in Celsius is linear at constant volume.
where m is the gradient of the function and c is the intercept the function makes with the vertical axis.

Furthermore, the function extrapolates to -273.15 oC at zero pressure regardless of the gas investigated (see graph above). Similar to the way a Celsius-based expression is derived from Jacques Charles’ experimental data, the Celsius-based formula for Gay-Lussac’s experiments is:
where p0 is the pressure of the gas at zero degrees Celsius and .
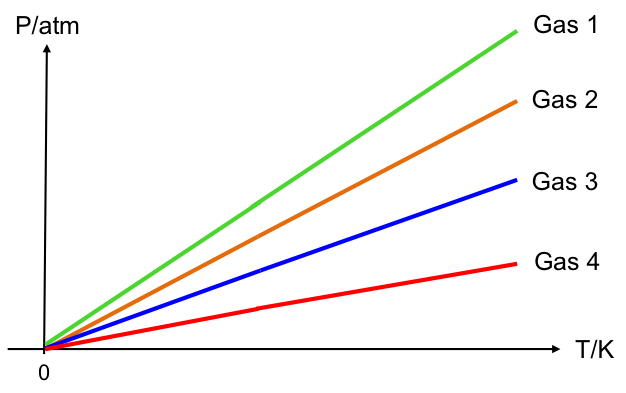
By changing the units of the horizontal axis from Celsius to Kevin (see diagram above), the relationship between pressure and temperature becomes directly proportional:
where k3 is the proportionality constant.
Eq11 is known as the Gay-Lussac’s law (or Amontons’ law), which states:
The pressure of a given mass of gas is directly proportional to its absolute temperature (K) at constant volume
Note that eq10 is also called the Gay-Lussac’s law (or Amontons’ law) with T in Celsius. Since p1 = k3T1 and p2 = k3T2, Gay-Lussac’s law can also be expressed as
Gay-Lussac also experimented on other properties of gases and discovered the law of combining volumes, which states:
Volumes of gases that react with one another are in the ratios of small whole numbers at a given pressure and temperature.
For example, two volumes of hydrogen gas react with one volume of oxygen gas to form two volumes of water:
