The vibrational molecular partition function is a measure of the number of thermally accessible vibrational energy states available to a single non-interacting molecule at a specific temperature.
Consider a diatomic harmonic oscillator with vibrational energies given by eq21: , where
. Substituting this equation into the vibrational component of eq257 gives:
Since for any temperature above absolute zero, we have
. Thus, the summation in eq290 can be evaluated as a binomial series,
, yielding:
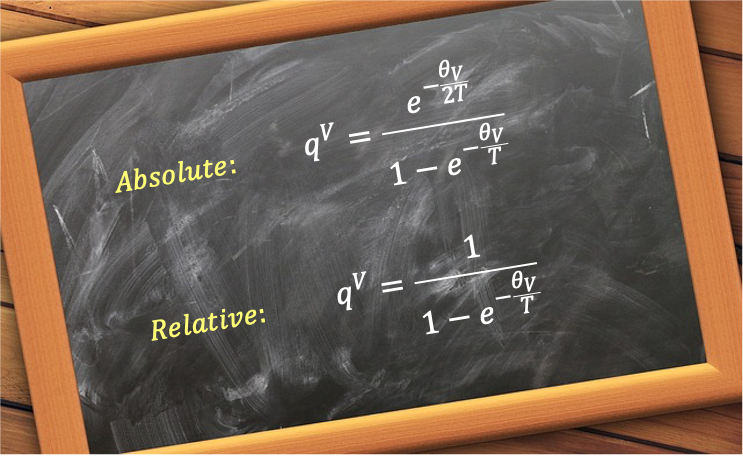
where the characteristic vibrational temperature is the analogue to the characteristic rotational temperature.
The vibrational partition function of eq291 expresses the exponential terms in terms of the absolute energy levels of the quantum harmonic oscillator, where the ground state energy is the zero-point energy . An alternative convention is to measure energies relative to the zero-point energy. In this case, the vibrational energy levels are given by:
This effectively sets the ground state energy to zero. The corresponding partition function becomes:
Applying the same binomial series summation results in:
Both eq291 and eq293 are valid expressions of the vibrational partition function. Eq293 is usually preferred because it simplifies calculations by omitting the constant zero-point energy for the ground state. However, when computing absolute energy quantities, such as the internal energy, the zero-point energy must be added back in.

Question
Show that eq291 and eq293 converges to the same expression for low frequency modes at high temperatures.
Answer
For low frequency modes at high temperatures, , and
can be approximated by the first-order Taylor series expansion:
. Eq291 reduces to
. At high
,
Similarly, eq293 becomes .
The convergence of the two vibrational partition function conventions for low-frequency modes at high temperatures reflects the emergence of classical behaviour where quantum corrections like zero-point energy become negligible. This demonstrates how statistical mechanics unifies quantum mechanics and classical thermodynamics.
For polyatomic molecules, each normal mode of vibration behaves approximately like a separate harmonic oscillator. Assuming that these vibrational modes are independent and separable, with negligible anharmonicity effects, the total vibrational energy of the molecule is the sum of the energies of the normal modes. It follows from eq292 that the total vibrational partition function is the product of the individual partition functions for each mode:
where for non-linear molecules,
for linear molecules, and
is the number of atoms per molecule.