A polytropic process is described by the formula , where
. The table below shows the association of different pV curves for ideal gases with the corresponding thermodynamic processes:
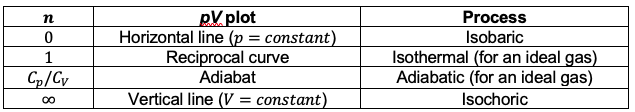

Question
Why is the projection (the curve obtained by representing the process in space on the
plane ) an adiabat when
and why is
a constant when
?
Answer
From eq44 and eq46, and
for an ideal gas. For adiabatic processes,
and hence,
. Furthermore,
. Since
,
. So,
which rearranges to , where
.
For the second why, . If
,
.