Alpha, beta and gamma rays are three fundamental forms of radiation emitted by unstable atomic nuclei during radioactive decay.
Alpha and beta rays
Ernest Rutherford in 1899 studied the radiation emitted from uranium compounds and observed that it was not uniform. In his experiment, he placed a small sample of natural uranium in form of uranium oxide in a metal container with a narrow opening so that the emitted radiation formed a directed beam (see diagram below). By introducing materials of different thicknesses in the path of the beam, he found that one component of the beam was easily stopped by a length of air, while a thin layer of aluminium greatly weakened the second component. The attenuated beam eventually reached a gold-leaf electroscope, whose leaves diverged, indicating that the second component was negatively charged.
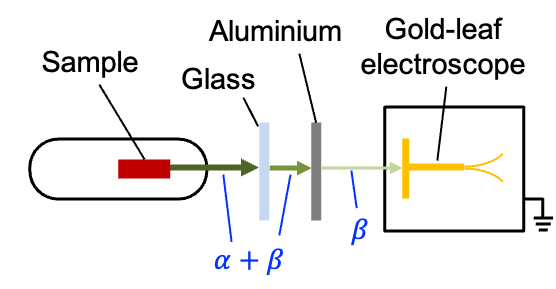
To test the nature of each radiation component, Rutherford inserted charged metal plates to create an electric field in the path of the beam. The weakly penetrating component was deflected only slightly and in a direction showing that it carried a positive charge, while the more penetrating component was strongly deflected towards the positive plate, confirming that it consisted of negatively charged particles.
From these results Rutherford concluded that uranium emitted at least two distinct types of radiation. He named the positively charged, weakly penetrating rays -rays, and the negatively charged, strongly penetrating rays
-rays.
The -rays were easily validated to be electrons using J. J. Thomson’s cathode ray techniques. Soon after, Rutherford and Frederick Soddy used electric and magnetic deflection methods, which were similar to those used in cathode-ray studies, to measure the charge-to-mass ratio
of
-particles. The precise identification of the
-particle as a doubly charged helium ion He2+ was ultimately confirmed by the Rutherford-Royds experiment. In this experiment, a beam of
-particles was allowed to enter a sealed chamber where they were trapped and then neutralised by capturing electrons from ionised residual gas. After sufficient accumulation, an electrical discharge through the gas produced the characteristic spectral lines of helium, demonstrating that
-particles are indeed helium ions.
Gamma rays
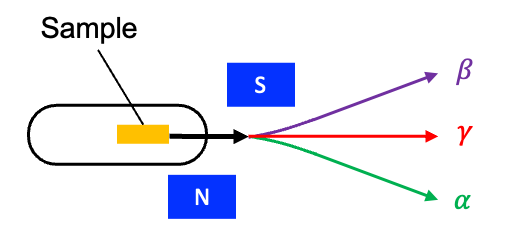
In 1900, French physicist Paul Ulrich Villard studied the radiation emitted from radium salts. As in Rutherford’s earlier experiment, the radiation was collimated and passed through an electric field and various barriers, including a thin layer of lead. The experiment was also repeated by passing the radiation through a magnetic field (see diagram below). Two components of the beam were deflected by the field, while a third, undeflected component was highly penetrating. Not long afterward, Rutherford recognised that the first two components matched the penetration and deflection patterns of alpha and beta particles. He then named the third, uncharged component –rays.

Question
What are the differences between X-rays and -rays?
Answer
Gamma rays and X-rays are both high-energy forms of electromagnetic radiation, but they differ in origin and energy range. Gamma rays are produced by nuclear reactions, whereas X-rays are generated by atomic electron processes, such as electronic transitions between inner atomic shells. The typical wavelength ranges of gamma rays and X-rays are <0.01 nm and 0.01–10 nm respectively.