In 1807, John Dalton, an English Chemist, introduced the atomic theory, which states that all elements are composed of indivisible atoms. He arrived at this conclusion by analysing data of different chemical reactions, particularly the proportion by weight in which chemical reactants combined to form products. As many of Dalton’s postulates are flawed (e.g. the idea that atoms are indivisible), the atomic theory is revised over the years to incorporate findings from newer experiments.

In the mid-1800s, the field of electrolysis saw a surge of experiments. Many scientists, including Michael Faraday, made contributions suggesting that the atom is not indivisible, but rather composed of smaller particles. In 1897, J. J. Thomson built one of the earliest mass spectrometers. He discovered a sub-atomic particle, now known as the electron, which has a mass 1700 times smaller than that of a hydrogen ion. Since atoms were known to be electrically neutral, Thomson conjectured that atoms must also be composed of positively charged matter with a magnitude equal to the electrons’ negative charge.
In the early 1900s, Earnest Rutherford conducted the Rutherford gold foil experiment, where a beam of He2+ ions (also known as -particles) were directed at a thin gold foil with the angular distribution of the particles recorded by a counter (see Fig I).
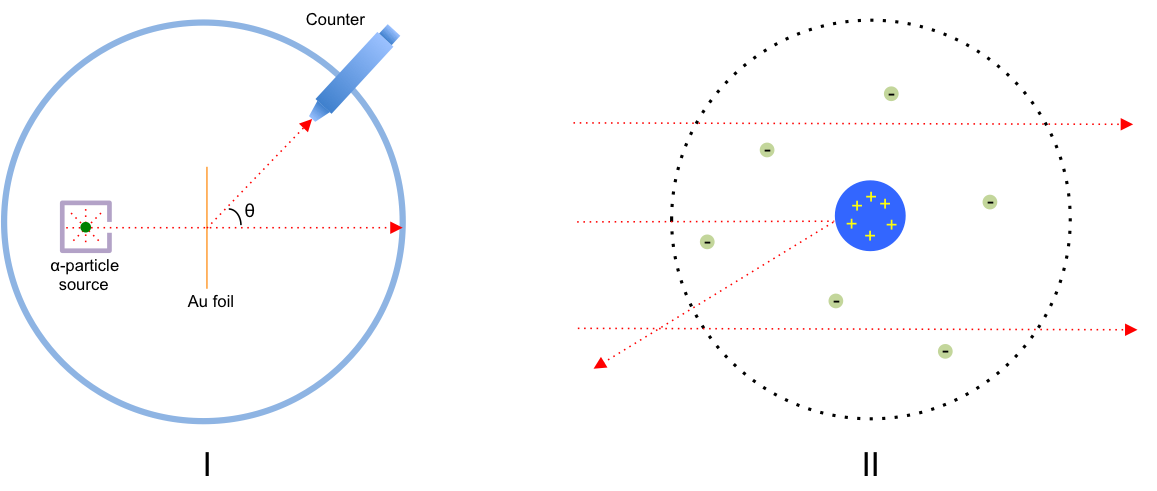
The results revealed that while most of the -particles passed straight through the gold foil, some were deflected at various angles of up to 1800, suggesting a strong repulsive force by positively charged matter within each gold atom. Rutherford knew from his calculations that if the positively charged matter were diffuse (i.e. distributed throughout the gold atom), its electric field would not be strong enough to deflect the
-particles. That led him to deduce that the positively charged matter must be concentrated in a small region of space in the gold atom. He proposed a model where an atom is composed of a dense and positively charged region in its center, surrounded by orbiting electrons (Fig II). Rutherford further proposed, after reviewing other scientists’ experiments, that the positively charged matter is composed of positively charged sub-atomic particles, which he called: protons.
Rutherford knew that his model was incomplete as the positively charged protons would repel each other apart if they were not held together by some other matter. Years later, he collaborated with Niels Bohr, a Danish physicist, and suggested that the protons in the center of an atom are held together by electrically neutral sub-atomic particles called neutrons. The existence of neutrons was eventually proven by James Chadwick, a British physicist, in 1932 and the combined mass of protons and neutrons is known as the nucleus of an atom.
We now know that protons and neutrons are composed of even more fundamental particles called quarks, which are in turn made of vibrating strings of energy.

Question
How did Chadwick discover neutrons and how do they prevent protons from repelling each other apart?
Answer
Chadwick directed -particles at beryllium, which emitted an unknown radiation that was not influenced by an electric field. The unknown radiation, upon hitting paraffin wax (a hydrocarbon), generated protons (see below diagram). He also found that the unknown radiation consisted of particles, each with mass similar to a proton (see below table), and concluded that they were neutrons.
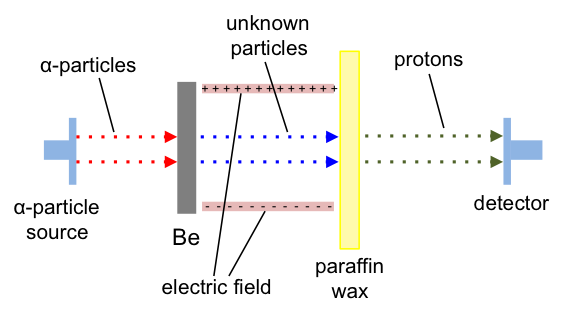
The force binding neutrons and protons in the nucleus is called the nuclear force, which is a residual force of the strong force that binds quarks to form neutrons. Although the nuclear force is a residual force and has a very short range, it is strong enough to bind protons and neutrons together.
|
Particle |
Mass (kg) | Relative mass | Charge (C) |
Relative charge |
|
Proton |
1.673 x 10-27 | 1 | 1.673 x 10-27 |
+1 |
|
Neutron |
1.675 x 10-27 | 1 | 0 |
0 |
|
Electron |
9.109 x 10-31 | 1.673 x 10-27 |
-1 |
