The surface area of particles plays a crucial role in determining the rate of chemical reactions. In a reaction involving a solid reactant, increasing the surface area of the solid enhances the reaction rate. Consider the following reaction:
A larger surface area of calcium carbonate allows more hydroxonium ions to collide with the carbonate molecules, facilitating more frequent and effective interactions.

As illustrated by the diagram above, a large cube of CaCO3 has six surfaces with a total area of 24 cm2 available for the hydroxonium ions to collide. However, if the cube is broken down into smaller cubes, the total surface area accessible to the hydroxonium ions increases to 48 cm2. This leads to a higher frequency of effective collision and therefore, a higher rate of reaction.

Question
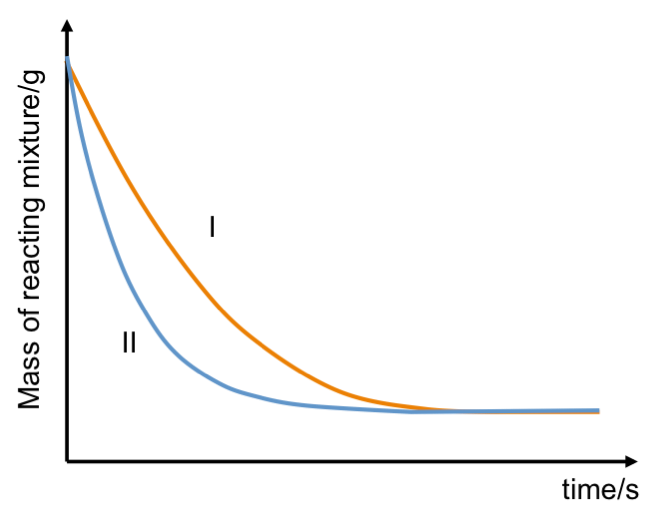
The masses of two reacting mixtures, each consisting of excess HCl and CaCO3, are monitored over time (see graph above).
Which curve corresponds to:
i) 50 g of CaCO3 chips?
ii) 50 g of finely grounded CaCO3?
When the reaction is repeated with 50 g of CaCO3 chips and H2SO4, the reaction stops, with most of the CaCO3 remaining unreacted. Why?
Answer
Curve II corresponds to the finely grounded CaCO3, which has a larger total surface area for reaction. The higher rate of reaction is deduced from the steeper gradient of curve II versus the gradient of curve I.
The reaction stops prematurely when H2SO4 is used, due to the formation of insoluble CaSO4, which coats the surfaces of the unreacted chips, preventing further reaction.