Electrolysis is a vital process in the extraction of metals, such as aluminium, from their ores, enabling the efficient separation of valuable elements through the application of electric current, which transforms metal ions into their solid form.
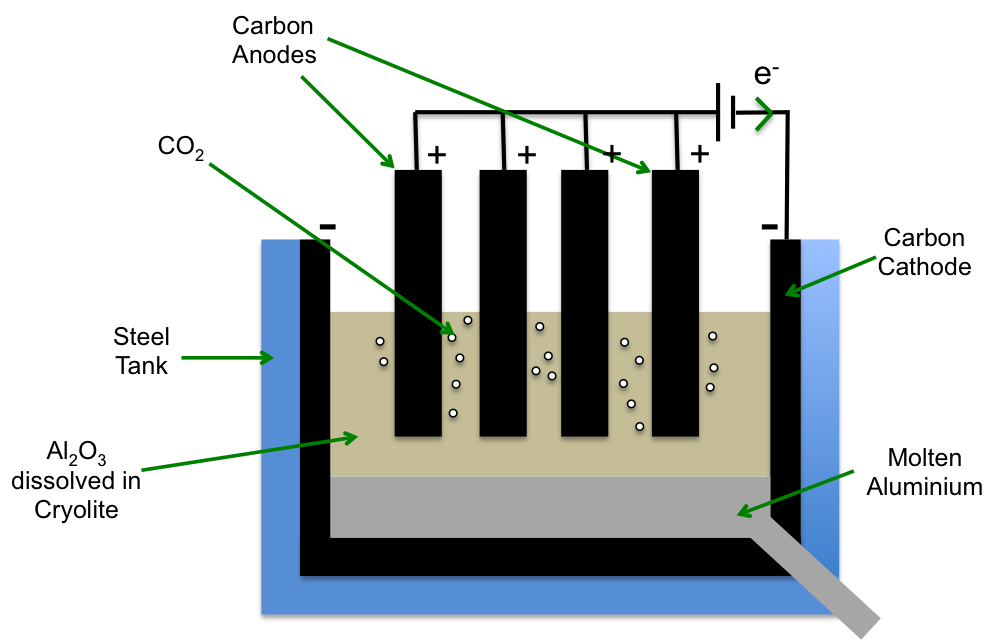
A theoretical way to obtain pure aluminium is to electrolyse molten Al2O3 (alumina). However, the melting point of Al2O3 is 2072 oC, making this method impractical. In the 1800s, French scientist Paul Héroult and American scientist Charles Hall developed a better method known as the Hall-Heroult process. In this process, Al2O3 is first dissolved in a solvent mixture of Na3AlF6 (cryolite) and AlF3 at about 1000 oC before being electrolysed to give pure aluminium.
Dissolution reaction:
Al2O3 (s) + 4[AlF6]3- (l) → 3[Al2OF6]2- (l) + 6F– (l)
Solid alumina is dissolved in cryolite and AlF3 at about 1000 oC to give an oxyflouridealuminate complex.
At the anode:
2[Al2OF6]2- (l) + 12F– (l) → 4[AlF6]3- (l) + O2 (g) + 4e–
O2 (g) + C (s) → CO2 (g)
The oxyflouridealuminate complex reacts with excess fluoride ions to give the hexafluoroaluminate complex and oxygen, which then oxidises the carbon anodes to yield carbon dioxide (note that at such high temperatures, the carbon anodes are no longer inert). The overall anode reaction is:
2[Al2OF6]2- (l) + 12F– (l) + C (s) → 4[AlF6]3- (l) + CO2 (g) + 4e–
At the cathode:
[AlF6]3- (l) + 3e– → Al (l) + 6F– (l)
The hexafluoroaluminate complex is reduced to give pure molten aluminium.
The overall redox reaction is
2Al2O3 (s) + 3C (s) → 4Al (l) + 3CO2 (g)
As the carbon anodes are oxidised to carbon dioxide, they have to be replaced over time.
next article: Applications of electrolysis: anodising
Previous article: Applications of electrolysis: refining of metals and electroplating
Content page of basic electrochemistry
Content page of Basic chemistry
Main content page