The Born-Haber cycle is a method to analyse reaction enthalpies, particularly to calculate the standard enthalpy change of lattice energy, ΔHlatto, which cannot be measured precisely via experiments. It is based on Hess’ law and was developed by the German scientists Max Born and Fritz Haber in 1916.
Born-Haber cycles are best represented in the form of energy diagrams. For example, if we want to calculate the standard enthalpy change of lattice energy of calcium fluoride ΔHlatto[CaF2(s)], we start by writing the equations for ΔHlatto[CaF2(s)] and the standard enthalpy change of the formation of calcium fluoride ΔHfo[CaF2(s)] :
Applying Hess’ law, we can combine the two equations into a cycle:
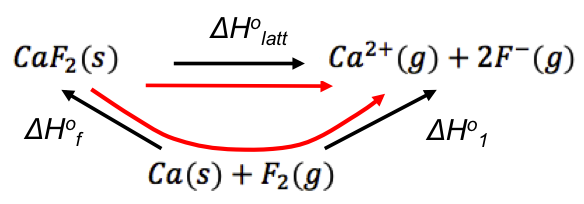
ΔH1o is composed of a few standard enthalpy changes. If we rewrite the cycle to include every standard enthalpy change (starting with the reactants of ΔHfo), we have the Born-Haber cycle for CaF2(s):
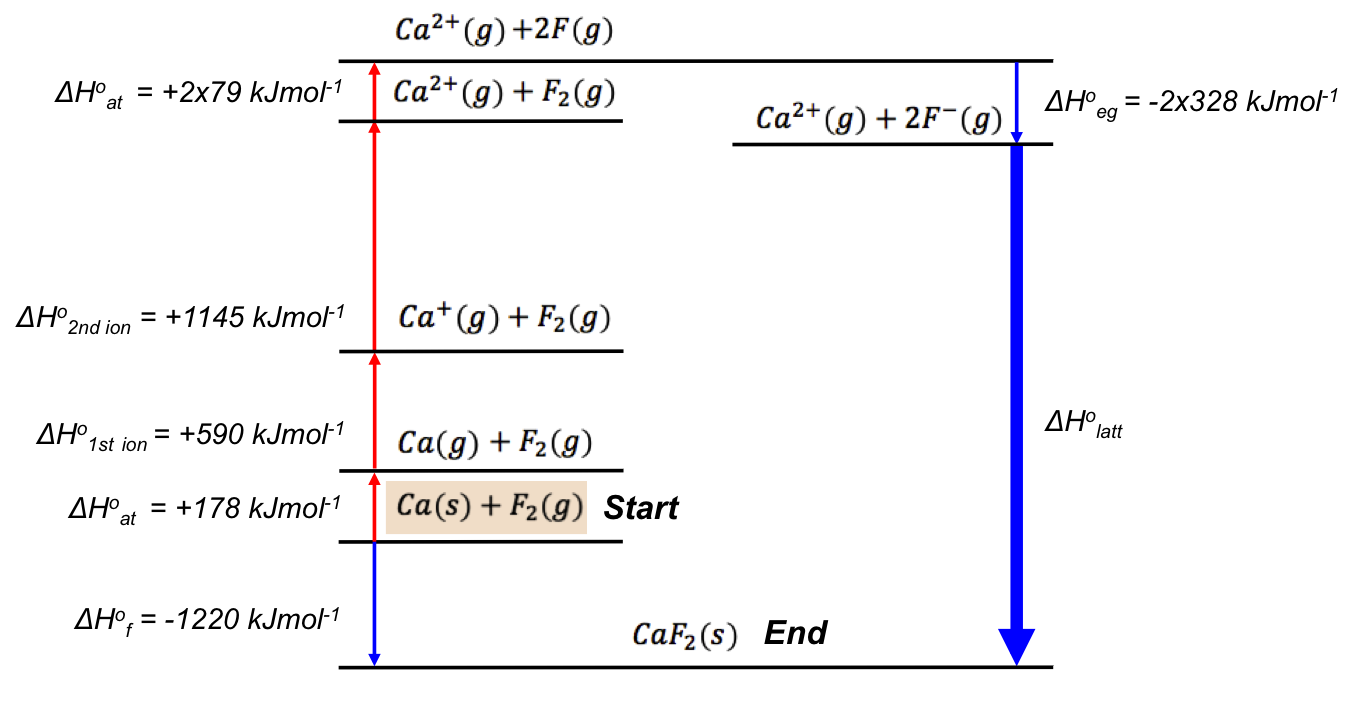
The Born-Haber cycle (also known as the Born-Haber energy diagram) reveals that ΔH1o is composed of:
-
- the standard enthalpy change of atomisation of Ca (ΔHoat),
- the standard enthalpy change of the first ionisation of Ca (ΔHo1st ion),
- the standard enthalpy change of the second ionisation of Ca (ΔHo2nd ion),
- the standard enthalpy change of atomisation of F2 (ΔHoat), and
- the standard enthalpy change of electron gain of F (ΔHoeg).
It is also evident that the sum of the magnitudes of each enthalpy on the left-hand side of the energy diagram is equal to that on the right-hand side, i.e.
The above computation shows that ΔHolatt for CaF2 can be +2635 kJmol-1 or -2635 kJmol-1, depending on its definition. Since we have defined the standard enthalpy change of lattice energy as an endothermic process (see eq7, where ΔHolatt is the change in enthalpy to break the bonds in one mole of a solid ionic compound and separate its gaseous ions to an infinite distance under standard conditions), ΔHlatto = +2635 kJmol-1.
In summary, the steps involved in generating the Born-Haber cycle are:
-
- Write the equations for the standard enthalpy change of lattice energy and the standard enthalpy change of formation of the ionic compound.
- Combine the two equations into a cycle using Hess’ law.
- Expand the cycle in a stepwise manner to include every standard enthalpy change.
- Draw the Born-Haber cycle energy diagram starting with the reactants of the standard enthalpy change of formation of the compound and ending with the ionic compound. There is no strict rule regarding what must be on the left or right side of the cycle, provided that the steps are shown sequentially and the arrow directions correctly reflect the signs of the enthalpy changes.
- Sum the magnitude of enthalpies on each side of the cycle and equate them to find ΔHlatto.

Question
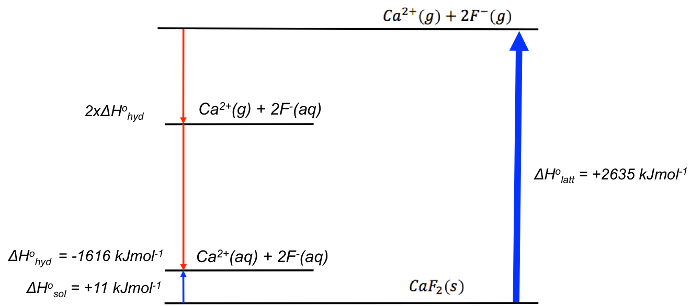
Calculate the standard change in enthalpy of hydration ΔHohyd for F– given that ΔHolatt [CaF2 ] = +2635 kJmol-1, the standard enthalpy change of solution of CaF2 ( ΔHosol [CaF2 ]) is +11 kJmol-1 and ΔHohyd [Ca2+] = -1616 kJmol-1.
Answer
With reference to the Born-Haber cycle below,