A catalyst is a substance that increases the rate of a reaction without itself being chemically changed at the end of the reaction. Catalysts can be categorised into two groups: homogeneous and heterogeneous.

A homogeneous catalyst is one that is in the same phase as the reactants, while a heterogeneous catalyst is one that is in a different phase as the reactants. Consider the decomposition of aqueous hydrogen peroxide to oxygen:
\rightarrow&space;O_2(g)+2H_2O(l)\;&space;\;&space;\;&space;\;&space;\;&space;\;&space;\;&space;\;&space;2)
The reaction occurs very slowly under normal conditions due to its high activation energy. If a homogeneous catalyst, e.g. hydrobromic acid HBr, is added, the reaction proceeds rapidly with the following two-step mechanism:
+Br^-(aq)+H^+(aq)\rightarrow&space;HOBr(aq)+H_2O(l))
+HOBr(aq)\rightarrow&space;O_2(g)+H_2O(l)+Br^-(aq)+H^+(aq))
In the first step, a molecule of H2O2, in the presence of H+, oxidises a bromide ion (oxidation state -1) to aqueous hypobromous acid HOBr (oxidation state of Br is +1), while it is reduced to water. In the second step, another molecule of H2O2 reduces the key intermediate HOBr back to Br– and H+, with itself being oxidised to a molecule of oxygen. If we add the two equations, we get eq2. You’ll realise that the hydrogen and bromide ions participate in the reaction but are not consumed in the overall process.


Question
Explain further why the two-step mechanism is energetically more favourable than the uncatalysed process?
Answer
The uncatalysed reaction requires two neutral hydrogen peroxide molecules to collide with the correct orientation, which has a relatively low probability of occurring. Furthermore, O–O bond cleavage and molecular rearrangement, both high energy processes, must take place within a single transition state before the products are form. This results in a high activation energy.
In contrast, in the catalysed pathway, the bromide ion is highly nucleophilic and interacts strongly with hydrogen peroxide. Since Br– is charged, electrostatic interactions and solvation effects help stabilise the transition state, lowering its energy. The intermediate HOBr formed is more reactive towards hydrogen peroxide than hydrogen peroxide is towards itself. This is because the O–Br bond is more polarised, making HOBr more electrophilic and allowing it to accept electrons more readily. Therefore, the reaction is broken into two elementary steps, each with a lower activation energy than the single, concerted uncatalysed process.
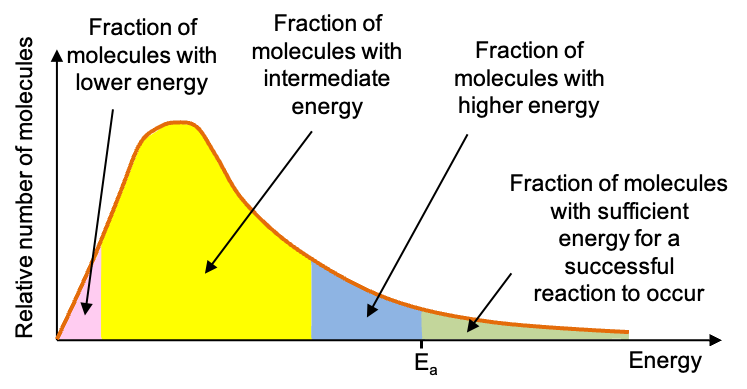
So, instead of the high activation energy route of two H2O2 colliding with each other to give oxygen, a suitably chosen catalyst offers a lower activation energy pathway of H2O2 colliding with a bromide ion, and subsequently with hypobromous acid, to produce oxygen. This is illustrated by the energy profile diagram above. With a lower activation energy, the rate of the reaction increases, as a larger proportion of molecules have sufficient energy to react.
The same reaction can be catalysed by a heterogeneous catalyst such as manganese dioxide. The mechanism for heterogeneous catalysis can be complex but normally involves the adsorption of reactants onto the surface of the catalyst, which changes the bond strengths of the reactants. The reactants, now being in close proximity to one another, react to form the products, which are desorbed or released from the surface of the catalyst (see diagram below).
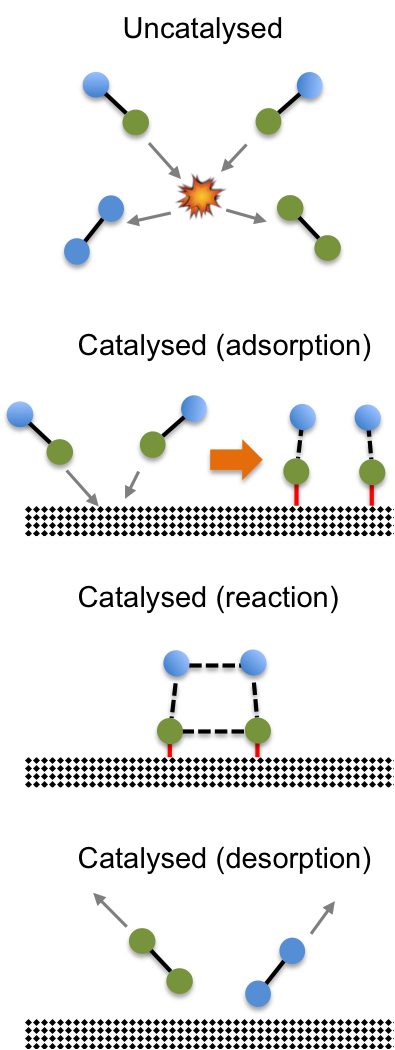
The catalyst, one again, participates in the reaction but remains chemically unaltered at the end of the reaction. Just like the example of the homogeneous catalyst, a heterogeneous catalyst offers an alternate reaction pathway for the reaction to occur. Instead of the high activation energy route of molecules colliding with one another to form products, a heterogeneous catalyst offers a lower activation energy pathway by allowing the molecules to be adsorbed onto its surface, where they react to form products.
In summary, a catalyst:
-
- Increases the rate of a reaction because it lowers the activation energy of the reaction by offering an alternate pathway for the reaction to proceed. For a reversible reaction, a catalyst increases the rates of both the forward and reverse reactions by the same factor because the activation energy is lowered for the reaction in both directions.
- Functions selectively, that is, it increases the rate of only certain reactions.
- Is required only in small amounts to increase the rate of reactions, as it is not consumed but reused in the process. Nevertheless, increasing the concentration of the catalyst further increases the rate of a single-step reaction, assuming that the reactants are in excess.
- May be inactivated by impurities over time.
- Can be denatured by heat if the catalyst is an enzyme.
Some common catalysts include:
-
- Iron in the Haber process for the manufacture of ammonia.
- Vanadium (V) oxide in the Contact process for the manufacture of sulphuric acid.
- Platinum, palladium or rhodium in catalytic converters.
- Nickel, platinum and palladium in the hydrogenation of alkenes to alkanes.
- Phosphoric acid on silica for the hydration of alkenes to alcohols.
- Concentrated acids for esterification.
- Biological enzymes produced by yeast in the conversion of sugar or starch to ethanol.