Charge balance equations are derived using the concept of electroneutrality, where the sum of positive charges equals to the sum of negative charges in a solution. Such equations are useful for analysing acid-base equilibria and formulating complex acid-base titration equations.
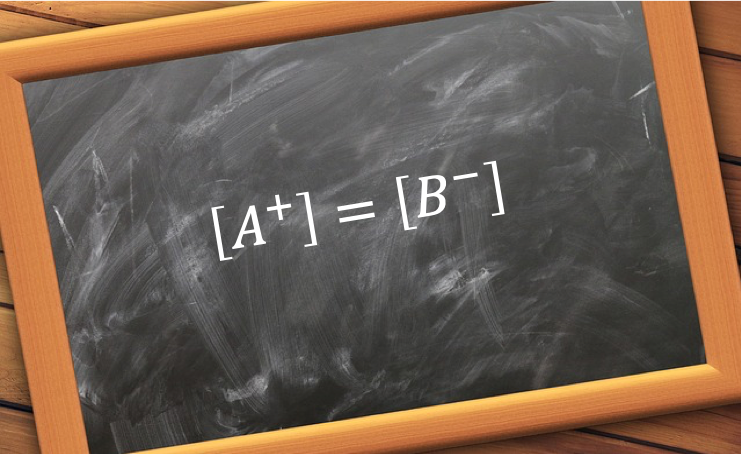
Consider a solution containing water, a strong acid of concentration Ca, and a strong base of concentration Cb, with the following equilibria:
To maintain electroneutrality, the sum of the number of moles of cations H+ and B+ must equal to that of anions A– and OH–. As the volume of the solution is common to all ions,
When formulating charge balance equations for aqueous compounds with multiple equilibria, we need to account for every charged species on the LHS of a particular equilibrium, which can be complicated. To avoid mistakes, we select equilibrium expressions where species on the LHS are neutral. For example, the equilibrium equations for a diprotic acid can be presented in the following ways:
Select the first and third equilibria, i.e., we can imagine part of the initial number of moles of H2A dissociating into Ha+ and HA–, with the remaining part of the initial number of moles of H2A dissociating into 2Hb+ and A2-. For the first equilibrium, the charge balance equation is
For the third equilibrium, the charge balance equation is
Combining eq31 and eq32,

Question
Write the charge balance equation for a triprotic acid.
Answer
Since,
we have,
