A system is the part of the universe being studied, while the surroundings are the remaining parts of the universe, with the two regions separated by a boundary. For example, if we are investigating the relationship between the volume and pressure of a gas using a J-shaped tube immersed in a constant-temperature bath, the system is the gas trapped in the left arm of the tube (see diagram below).
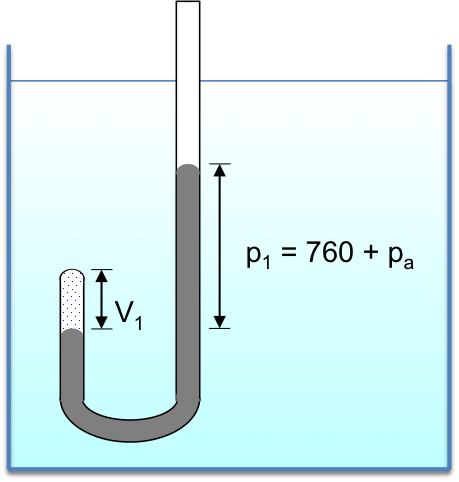
The surroundings include the entire length of mercury in the tube and the constant temperature bath. The boundary consists of the wall of the J-shaped tube and the mercury meniscus in the left arm of the tube.
We need not consider the air in the right arm of the tube, the wall of the container of the bath and the air in the atmosphere since the system is in thermal equilibrium with the mercury and the bath.
The example mentioned above describes a closed system, where energy, but not matter, can be transferred between the system and its surroundings. This implies that the boundary of a closed system is thermally conductive. An open system is one in which both energy and matter can be transferred between the system and its surroundings. The boundary in this case is either physically permeable or notional. If neither energy nor matter can be transferred between the system and its surroundings, the system is called an isolated system, where the boundary is both thermally non-conductive and impermeable—that is, an adiabatic boundary.