An electric dipole is a pair of equal and opposite electric charges, and
, separated by a small distance
. The electric dipole moment
(also denoted by
) is a measure of the strength and orientation of the electric dipole and is defined as:
where is the position vector of the corresponding charge measured from the origin, which is usually taken at the midpoint between the charges.
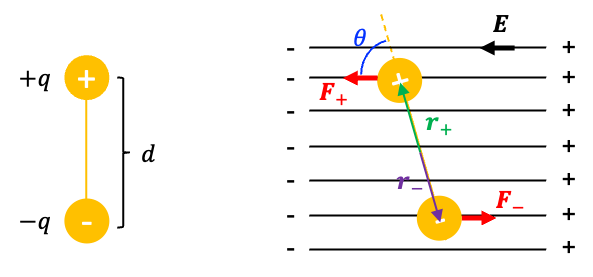
The SI unit of electric dipole moment is the Coulomb-meter (C·m), although the Debye (D) is more commonly used in practice (1D » 3.33 x 10-30 C·m)

Question
Is the electric dipole a vector?
Answer
The electric dipole is a physical configuration of two equal and opposite charges, not a vector. However, the associated electric dipole moment is a vector quantity. By physics convention, its direction points from the negative charge to the positive charge (note that in chemistry, the dipole direction is taken from the less electronegative atom to the more electronegative atom).
When an electric dipole is placed in an external electric field (see diagram above), it experiences a torque
that tends to rotates the dipole so as to align it with the field, thereby lowering its potential energy. The torque is defined as the product of the component of a force
normal to the axis of rotation and the distance
from the origin to the point of application of the force (
or equivalently
). For a spatially uniform electric field,
and
define a plane, and
is perpendicular to this plane, with its direction given by the right-hand rule. Therefore, the magnitude of the torque is
where is the angle between the rotating axis and the force, which is equivalent to the angle between
and
.
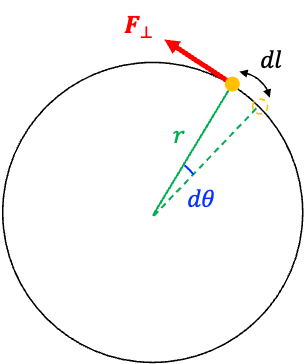
For a rotating system (see diagram above), the work done is given by
Since and the change in potential energy
is the negative of the work done by the field,
Substituting eq352 into eq353 yields:
Because the force exerted by the electric field rotates the dipole towards a lower potential energy, . To satisfy this condition, as
changes from
to
during the rotation, we define
when the dipole is perpendicular to the field (
), giving
where and the negative sign arises naturally to ensure that
(
is positive for
), with the corresponding potential energy in vector form being
The electric dipole moment is used to determine electronic transition probabilities in atoms and molecules.