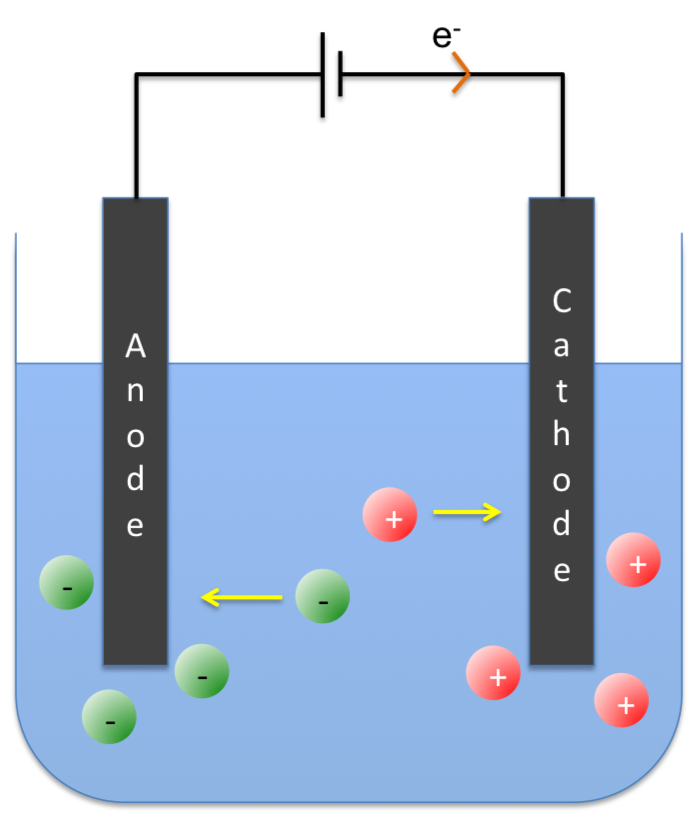
Faraday’s first law of electrolysis states that the amount of chemical change produced at an electrode is proportional to the quantity of electricity used.
Faraday conducted an experiment by passing a known quantity of electricity through an electrolytic cell containing an aqueous metallic salt. After determining the amount of chemical change by weighing the electrodes before and after the experiment, and repeating the experiment with different quantities of electricity, he found that:
The amount of chemical change produced at an electrode is proportional to the quantity of electricity used
Mathematically, we have:
where W is the gain or loss in weight of an electrode (i.e. the amount of substance produced at an electrode) and Q is the quantity of electricity passed through the electrolytic cell.
Since Q = It, where I is the current and t is time,
Faraday subsequently conducted the experiment using different electrolytes and obtained the same proportional relationship between W and Q, albeit with different proportionality constants:
He named the proportionality constant Z the electrochemical equivalent of a substance and defined it as the gain or loss in weight of an electrode during the experiment when a current of one ampere was passed through the electrolytic cell for one second.
