The standard enthalpy change of solution, ΔHsolo, is the change in enthalpy when one mole of a solute dissolves in a solvent to form an infinitely dilute solution under standard conditions. This means that we need to dissolve the solute in excess solvent until there is no change in the energy absorbed or released by the system.

Some examples are:
Since KOH(aq) and HCl(aq) are fully dissociated in water, we can also write the above equation as:
ΔHsolo can be positive or negative. Compounds with large positive ΔHsolo are relatively insoluble.
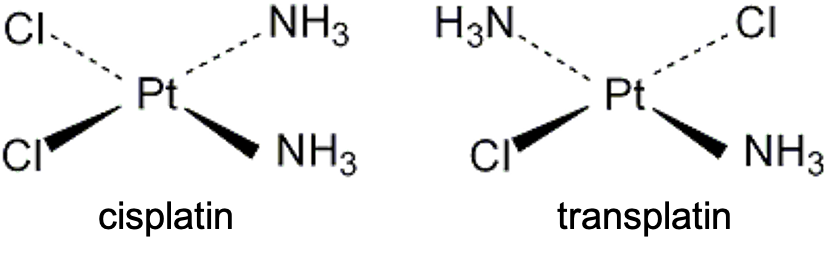

Question
With reference to the diagram above showing the molecular structures of cisplatin (a chemotherapy drug) and transplatin, deduce which has a less positive standard enthalpy change of solution (in water), and hence, is more soluble in water.
Answer
According to Hess’s law,
where and
are the standard enthalpy changes of sublimation and hydration respectively.
Both cisplatin and transplatin have square-planar molecule geometry. In the cis configuration, the two chloride ligands are adjacent to each other, and the two ammonia ligands occupy the remaining adjacent positions. Because Cl and NH3 have different electronegativities, their bond dipole moments do not cancel. This creates a polar molecule with a significant net electric dipole moment. In the trans configuration, identical ligands are positioned 180° apart. The bond dipoles of the two Pt–Cl bonds cancel each other, as do those of the two Pt–NH₃ bonds, resulting in a non-polar molecule with a net electric dipole moment of zero. Since water is a polar solvent, it interacts much more favourably with polar molecules. The stronger dipole–dipole interactions between water and gaseous cisplatin result in a more exothermic (more negative) standard enthalpy change of hydration than for transplatin.
In the solid phase, transplatin molecules are more symmetrical and can pack more efficiently into the crystal lattice. This more efficient packing increases intermolecular attractions in the solid, so the standard enthalpy change of sublimation of transplatin is expected to be more positive than that of cisplatin.
Combining the two terms, cisplatin has a more negative and a less positive
. Therefore, its standard enthalpy change of solution is less positive than that of transplatin, consistent with cisplatin being more soluble in water.