There are possibly as many standard enthalpy changes as there are types of reactions. Some common ones other than those mentioned in previous articles include:
-
- Standard enthalpy of hydrating an anhydrous salt (not to be confused with standard enthalpy of hydration), e.g.
-
- Standard enthalpy of precipitation, e.g.
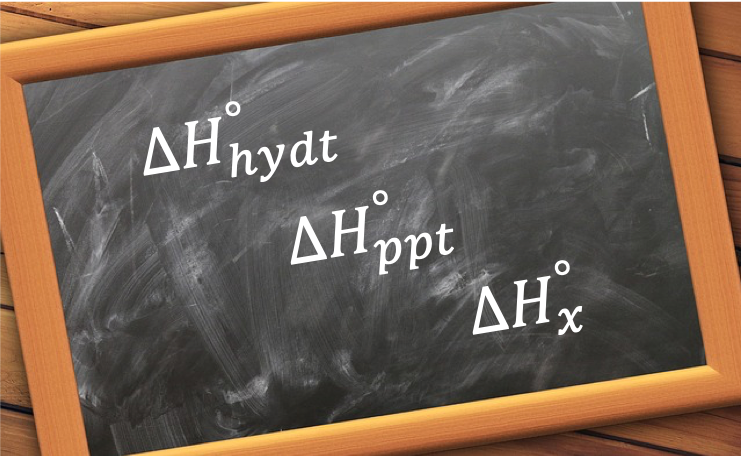

Question
Calculate the enthalpy of precipitation of PbBr2 when 150.0 mL of 0.500 M Pb(NO3)2 is added to 80.0 mL of 1.000 M NaBr in a calorimeter with the temperature rising from 298.15 K to 299.28 K (assuming that the solution’s specific heat capacity is 4.200 Jg-1K-1 and its density is 1.0 g/ml).
Answer
0.0800 moles of NaBr precipitates 0.0400 moles of PbBr2. Using eq5 from a basic level article,