Spectroscopy is the scientific study of the absorption and emission of electromagnetic radiation by matter.
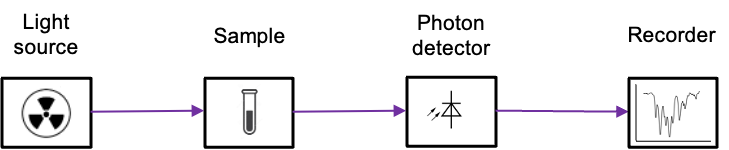
The instrument used for analysing these interactions is known as a spectrometer. A typical spectrometer consists of a light source that produces a beam with a range of electromagnetic frequencies (see diagram below). This beam passes through the sample, where it may be absorbed. The remaining transmitted radiation is detected and recorded, forming an absorption spectrum.
The absorption of a photon of energy causes a chemical species to undergo a transition from a lower energy state
to a higher energy state
. Different magnitudes of
elicit various transitions. These transitions can occur between rotational states, vibrational states, electronic states, and even nuclear spin states of a chemical species (see diagram below).
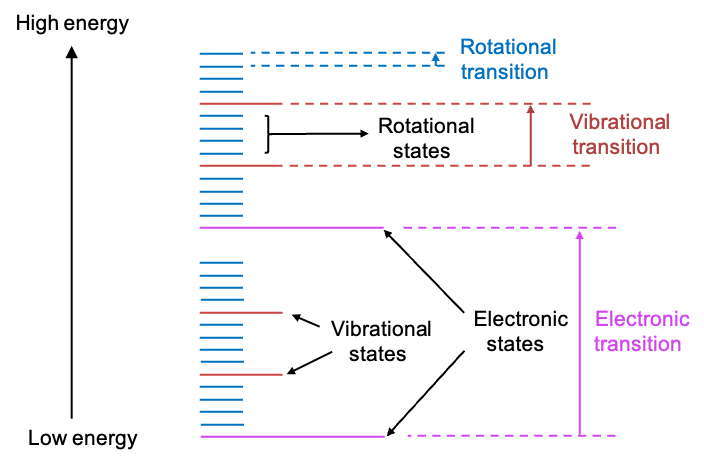
A variety of spectroscopic methods are used to analyse these transitions. For example, infrared (IR) spectroscopy focuses on transitions between vibrational states, rotational spectroscopy analyses transitions between rotational states, Raman spectroscopy also studies transitions between both vibrational and rotational states, and nuclear magnetic resonance (NMR) spectroscopy studies transitions between different nuclear spin states.