An ionic equation is a simplified version of a chemical equation that involves ions.
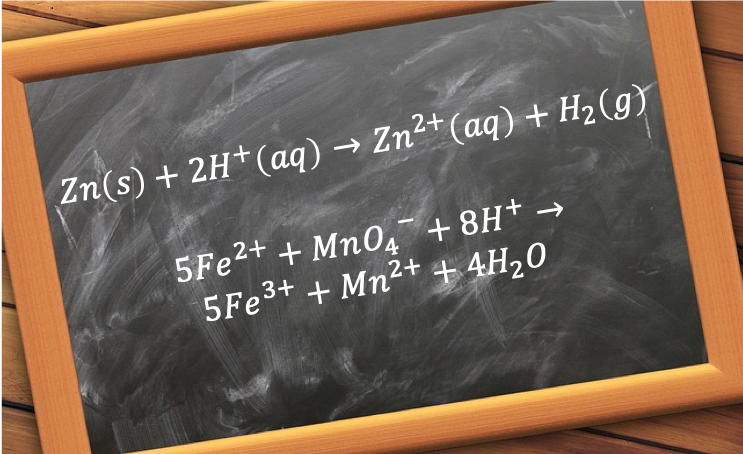
The construction of an ionic equation is usually accomplished in three steps, which can be illustrated using the example of the reaction between solid zinc and a solution of sulphuric acid:
Step 1: Write the full equation.
Step 2: Rewrite the equation such that the aqueous species are in their dissociated forms.
Step 3: Remove ions that appear on both sides of the equation, as these ions are known as spectator ions.
The purpose of writing an ionic equation is to highlight the chemical species that are playing an active role in a reaction. In the reaction above, the active species are Zn(s) and H+(aq) for the reactants, and Zn2+(aq) and H2(g) for the products, with SO42-(aq) playing the ‘inactive’ or spectator role.

Question
Elaborate further on why SO42- plays an inactive role in the reaction.
Answer
The sulphate ion appears unchanged on both sides of the equation. It neither gains nor loses electrons and does not take part in the redox process. Therefore, it plays an inactive role in the reaction and is called a spectator ion.