IR drop in electrochemistry is the voltage drop due to the resistance of the analyte (and the internal KCl solution if a calomel or AgCl electrode is used) in a close circuit.
In the previous article regarding pH measurement, which involves potentiometry, no net current flows in the circuit. When a current flows, e.g. in coulometry and electrogravimetry, we have to consider two effects:
-
- IR drop
- Overpotential
We can represent IR drop as an internal resistance of a battery in the following circuit diagram:
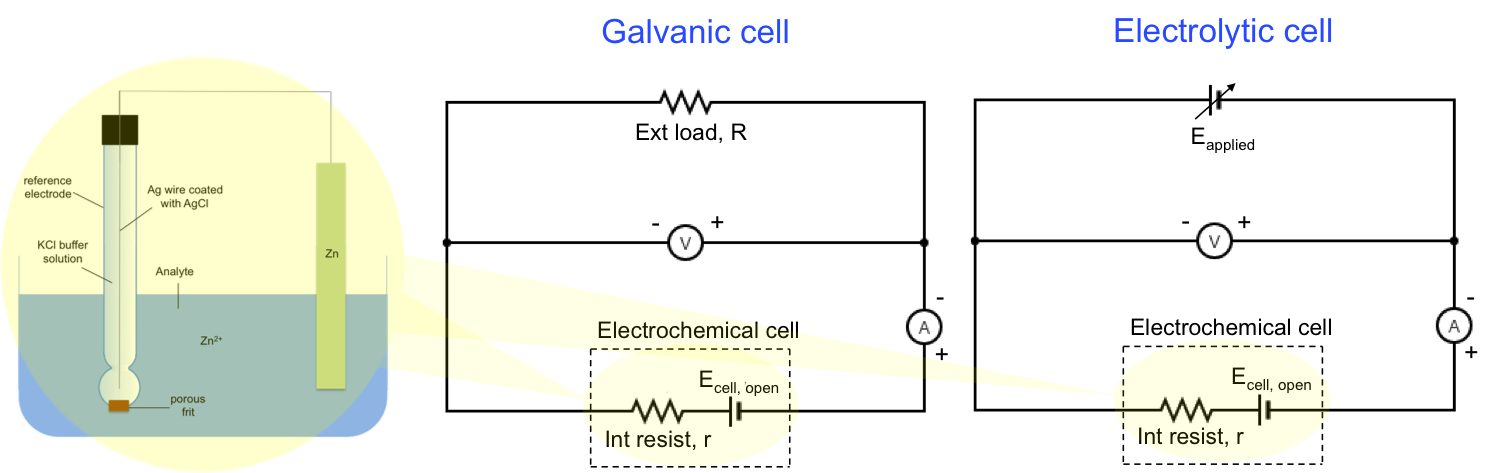
The general equation for a galvanic cell is:
where Ecell, open = ER – EL.
Eq32 when applied to a Zn electrochemical cell gives the following values:
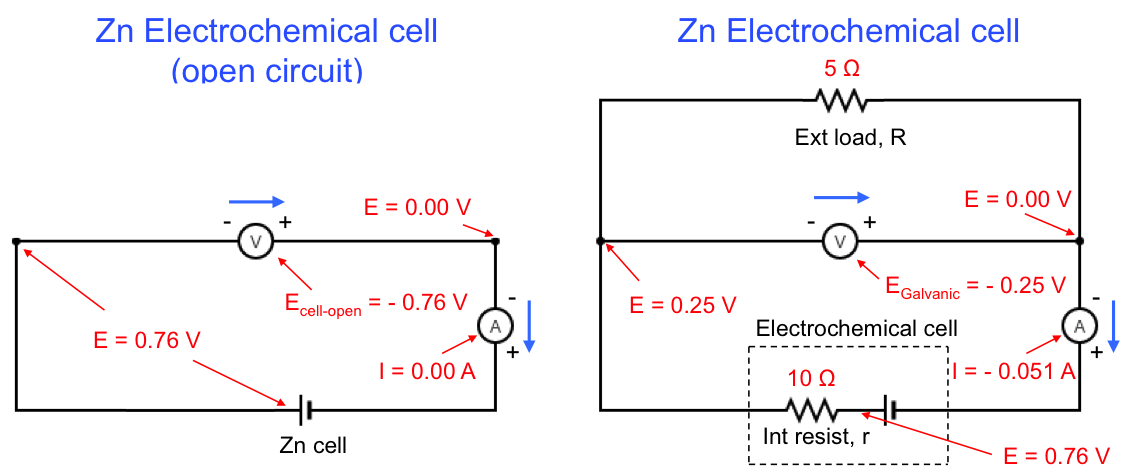
Ecell, open and Egalvanic for the above example are recorded as negative values by the voltmeters because of the way the terminals of the voltmeters are connected to the respective circuits. From eq32, is always less than
.
For an electrolytic cell,
If no current is flowing in the electrolytic cell, eq33 reduces to Eapplied = Ecell, open. For electrolysis to proceed, . For example, the variation of Eapplied for the electrolytic reduction of Zn2+ is illustrated by the following diagram:
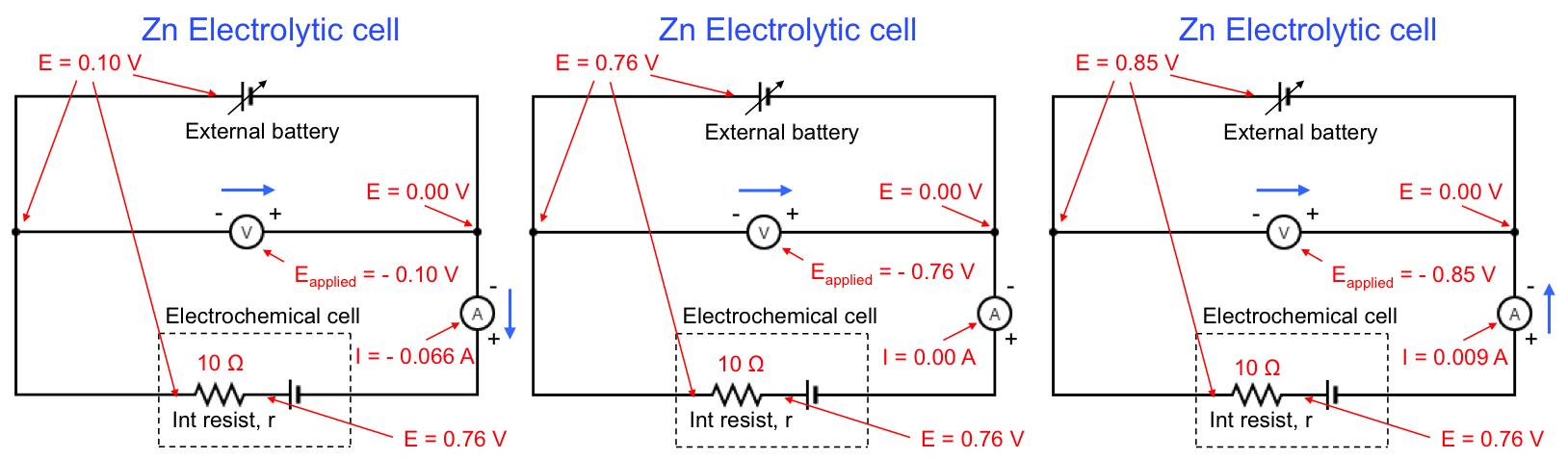
Once again, Eapplied for the above example is recorded as a negative value by the voltmeter because of the way the voltmeter is connected to the circuit.

Question
With reference to the above electrochemical cell that is composed of a saturated AgCl reference electrode, a Zn working electrode and a 0.5 M Zn2+ electrolyte at rtp, what external voltage must be applied to generate an electrolytic current of 3 mA if the internal resistance is 10 Ω. What is the IR drop?
Answer
Using the Nernst equation,
Using eq33,
Therefore, the IR drop is
