Ligand field theory describes the electronic and magnetic properties of coordination complexes resulting from the interaction of peripheral donor atoms with -orbitals of the central metal atom.
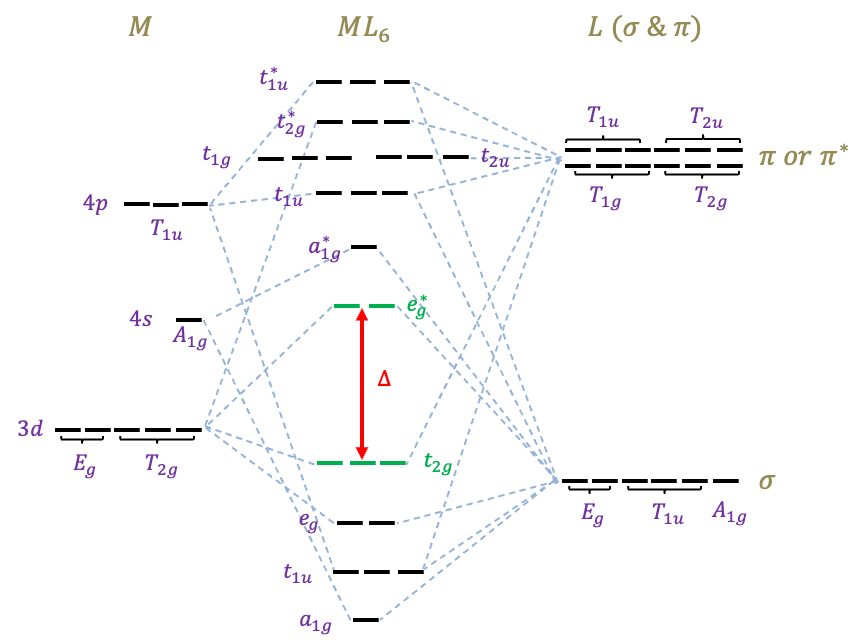
Molecular orbital (MO) diagrams of -metal complexes (see above diagram for an example, and read the previous article on how it is constructed) provide the theoretical foundation for the ligand field theory. In the context of an octahedral complex, the energy separation between
and
, known as the ligand field splitting parameter
, is a crucial aspect of this theory. This energy difference is responsible for distinguishing the electronic and magnetic properties among different complexes. The magnitude of
for a complex with a specific metal is dependent on the type of ligands. Ligands (e.g.
and
) that interact strongly with the metal orbitals are called strong-field ligands, while ligands with weak interactions (e.g.
and
) are known as weak-field ligands.

Question
Why does the interaction of a strong-field ligand with the metal lead to a larger ?
Answer
A ligand like is a
-acceptor ligand. It has low-lying vacant
orbitals that can overlap with the
orbitals of the metal ion. This
-back-donation from metal
orbitals to ligand
orbitals lowers the energy of the
MOs and therefore increases the energy separation between
and
. The stronger the
-acceptor ability of the ligand, the greater the splitting of the
orbitals.
Consider the following two cases:
-
- Strong-field:
is significantly large, making it energetically advantageous to fill the
orbitals before the
orbitals.
- Weak-field:
is small, which makes it energetically preferable to fill the
orbitals before the
orbitals are entirely occupied.
- Strong-field:
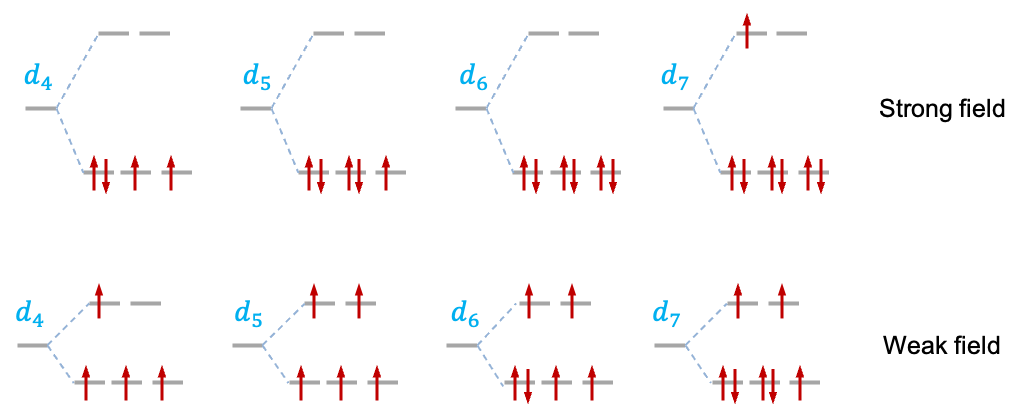
The diagram below shows the ground state configuration of -complexes, where
, for both the strong-field and weak-field cases. For instance, it is energetically favourable for a
-complex to adopt the
configuration if
is large. When
is small, the more stable configuration is
, where the electrons are in separate orbitals with parallel spins. Complexes with configurations of 3 or more unpaired spins are classified as high-spin complexes, while configurations of less than 3 unpaired spins are known as low-spin complexes.
The above MO diagram is useful for studying charge transfer transitions of an octahedral complex. Charge transfer transitions occur between MOs that are mostly metal in character and those that are mostly ligand in character. These transitions depend on the type of ligand: they occur only when the metal is bound to ligands that are -donors or
-acceptors. For instance, the transition from
to
is known as a ligand to metal charge transfer (LMCT). If we’re interested in studying
transitions, which are electronic transitions that occur between MOs that are mostly metal in character, a correlation diagram comes in handy.