A metal displacement reaction is one in which a less reactive metal in a compound is replaced by a more reactive metal.
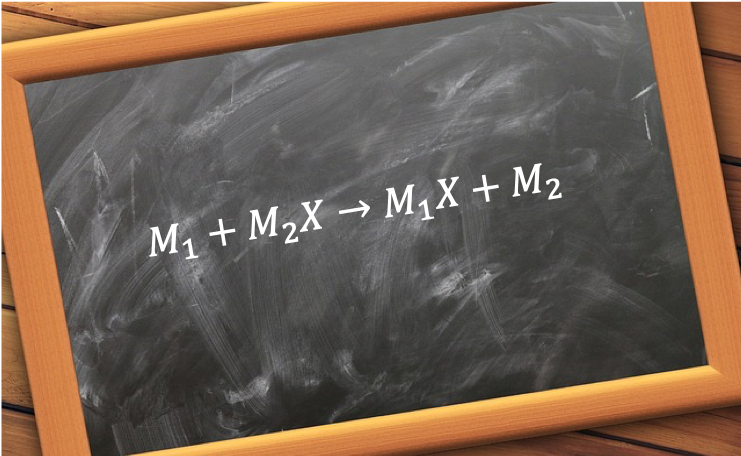
The displacement occurs because the overall energy of the system is lowered, making the forward reaction energetically favourable. Essentially, the more reactive metal has a greater ability to donate electrons, which drives the displacement reaction. For example, the thermite reaction (also known as the Goldschmidt process) is used to prepare small quantities of metallic iron:
2Al (s) + Fe2O3 (s) → Al2O3 (s) + 2Fe (s)
Question
What do you observe when you place a strip of magnesium in a beaker containing dilute aqueous copper sulphate?
Answer
The blue solution turns colourless with pink deposits at the bottom of the beaker.
Mg (s) + CuSO4 (aq) → MgSO4 (aq) + Cu (s)