Robert Millikan conducted an experiment in 1909 to measure the charge of an electron. He devised an apparatus to observe electrically-charged droplets of oil between two horizontal plates that had a potential difference of several thousand volts applied across them.
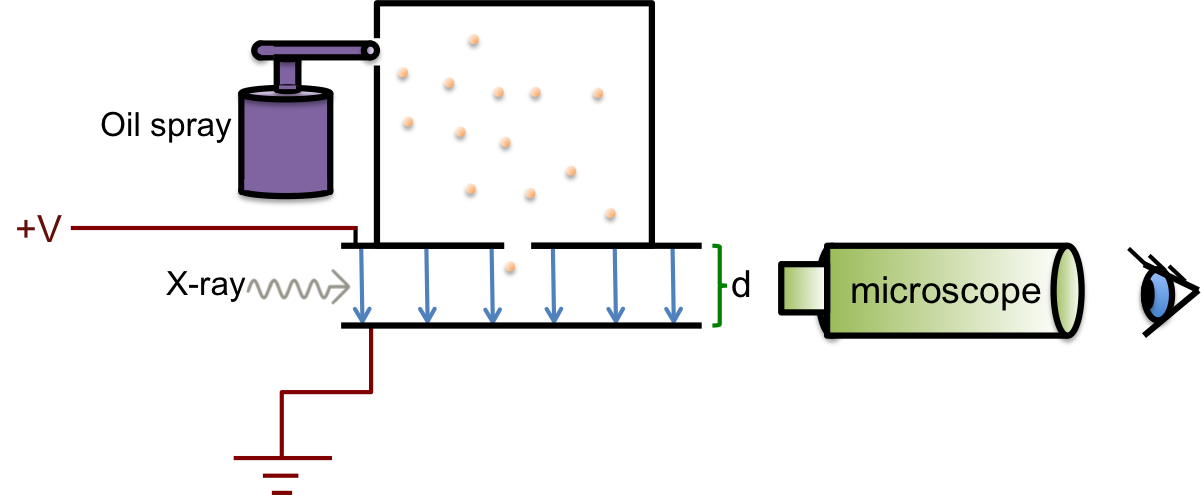
The oil droplets were sprayed from an atomiser into an upper chamber. In the space between the plates, the air were ionised by X-rays, causing the oil droplets to acquire electrons and become negatively charged as they fell and traversed through the air between the plates. Millikan then performed a few tasks and observed the motion of the oil droplets through a microscope.