The chemical shift δ in nuclear magnetic resonance spectroscopy is a measure of the spin transition frequency of a nuclide with respect to a standard nuclide. It is dependent on the identity of the nuclide and the chemical environment of the nuclide. From the previous article, we learned that the concept of nuclear magnetic resonance involves analysing resonance signals of nuclides by varying an alternating electromagnetic frequency v at a constant external magnetic field B, or by varying an alternating B at a constant v. According to Lenz’s law, a changing B induces an opposite magnetic field in an electron-filled system like an atom. The magnitude of the induced magnetic field is dependent on the atom’s electron density, which is influenced by neighbouring atoms. Since this induced magnetic field is directionally opposite to the external magnetic field, the effective external magnetic field felt by the nucleus is:
where σ is a constant called the shielding constant, which varies according to the chemical environment of an atom.
For example, 1H in the aldehyde functional group RCHO experiences a larger Beff than 1H in CH4. This is because the electropositive carbonyl carbon reduces the electron density around the hydrogen nucleus (deshield), resulting in a lower induced magnetic field when the 1H nucleus is exposed to an external magnetic field. We say that the shielding constant of 1H in the aldehyde functional group of RCHO is smaller than that of 1H in CH4, or that 1H in the aldehyde functional group of RCHO is relatively more deshielded than 1H in CH4 (note that all 1Hs in CH4 are chemically equivalent).
Substituting eq3 and the Planck relation in eq2 of a previous article, we have
Eq4 shows that if B is fixed (or if v is fixed), the radiofrequency v (or the external magnetic field B) needed to stimulate a nuclear spin transition is dependent on the gyromagnetic ratio of the nuclide and the shielding constant. The remaining task is to set up a convenient measure, i.e. a scale, to represent the resonance frequencies for different nuclides. This scale, known as the chemical shift δ, is:
where vo is the resonance frequency of a reference nuclide—tetramethylsilane (TMS) with the molecular formula Si(CH3)4 is commonly used as a reference in 1H-NMR.
The reason why the fractional change term is multiplied by 106 is that the numerator is expressed in hertz, while the denominator is in megahertz. For example, the resonance frequency of 1H in TMS at a particular fixed B is 300 MHz, and that the resonance frequency of a 1H in a sample that we are investigating is 300.0003 MHz at the same B. We would expect 2 peaks in the NMR spectrum, one for the chemically equivalent 1Hs in TMS at δ = 0 (substituting v = vo in eq5), and the second peak for 1H-sample at:
Since is 1 part per million, the units for δ is ppm. Therefore, instead of plotting the horizontal axis of the NMR spectrum in Hz or MHz, we plot it in δ, which has a simpler value. A typical low-resolution spectrum looks like this:
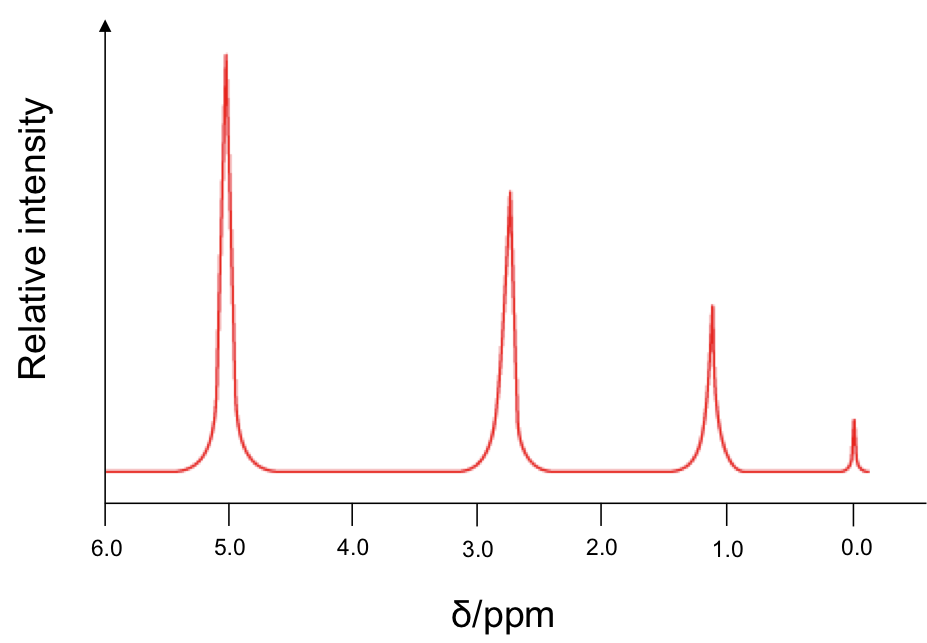
The horizontal axis is plotted such that the more heavily shielded nuclei appear to the right of the spectrum. The small peak at 0.0 ppm corresponds to 1H from TMS. The areas under the peaks reflect the relative number of chemically equivalent 1H for the respective chemical shift.
Some common chemical shifts of 1H nuclides are as follows:
| 1H nuclides | δ/ ppm |
| TMS | 0.0 |
| RCH3 | 0.7 – 1.2 |
| RCH2R | 1.1 – 1.5 |
| R3CH | 1.6 – 2.0 |
| ArCH3, ArCH2R, ArCHR2 | 2.3 – 2.7 |
| CH3COR | 2.0 – 3.0 |
| -OCH3, -OCH2R, -OCHR2 | 3.0 – 4.0 |
| RaCHbX where a = 0,1,2 b = 1,2,3 X = Cl, Br | 3.0 – 4.2 |
| RNH2 | 1.0 – 4.8 |
| ROH | 1.0 – 6.0 |
| ArH | 6.5 – 9.0 |
| RCHO | 9.5 – 10.5 |
| RCO2H | 10.0 – 13.0 |
13C also has a nuclear spin of ½ and is NMR active. However, a large sample is needed for analysis, as its natural abundance is about 1.1%. The typical resonance frequency of a 13C nuclide is a quarter of that of a 1H nuclide, which allows 13C spectra to be generated separately from 1H spectra. The chemical shift of 13C ranges from 0 ppm to 220 ppm. Unlike a 1H spectrum, the areas under peaks in a 13C spectrum are not a reliable indication of the relative number of carbons. This is because some carbon signals, e.g. carbonyl carbons, are weaker than other carbon signals, e.g. methyl carbons.

Question
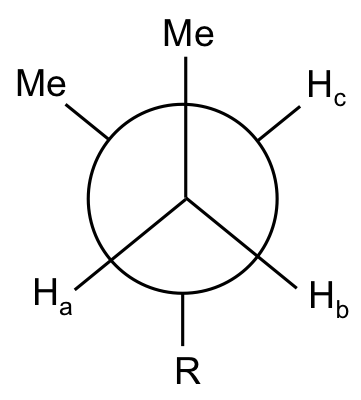
Do the protons on the marked carbon have the same chemical shift?

Answer
The two protons, Ha and Hb, have different chemical shifts, as they are not equivalent (see the Newman projection below):