The diverse chemical structures of common pH indicators, ranging from simple organic dyes to complex conjugated systems, play a crucial role in their ability to visually signal changes in acidity and alkalinity.
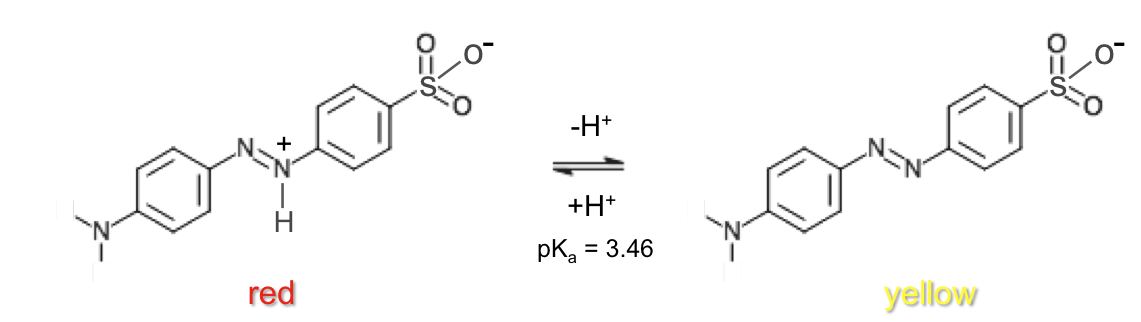
Methyl orange is a commonly used indicator. At low pH, protonation of the nitrogen at the para position of the benzenesulphonate moiety is preferred over protonation of the negatively charged oxygen in the sulphonate group, as this confers resonance stabilization (benzenoid-quinonoid tautomerism) to the acid form of methyl orange.
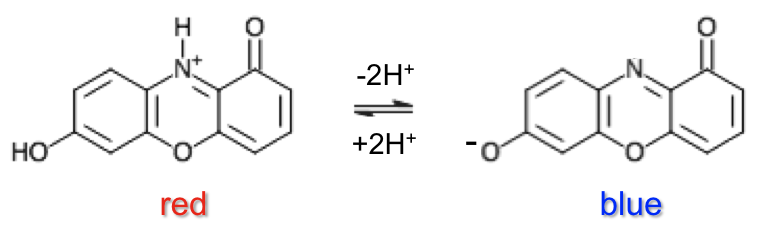
Litmus, extracted from lichen, is processed and applied onto filter paper. It has a pH range of 4.5 to 8.3 and contains the chromophore, 7-hydroxyphenoxazone, which occurs in the following states:
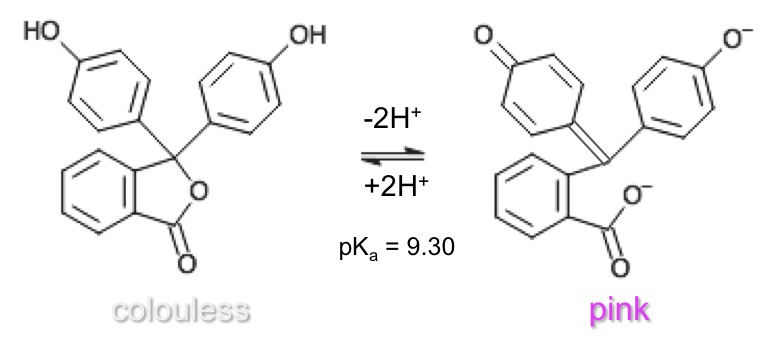
Phenolphthalein has three pKa values with four different forms. However, stoichiometric points of titrations are most commonly monitored with the middle pKa value of 9.30, which involves the lactone and phenolate (dianionic) forms:
Thymol blue has two transition range with pKa1 = 1.65 and pKa2 = 8.96.

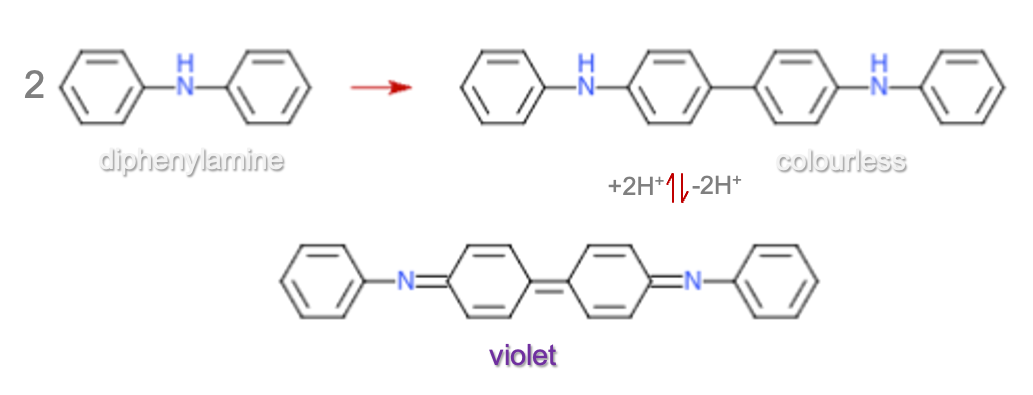
Diphenylamine acts as a redox indicator in titrations involving potassium dichromate (K₂Cr₂O₇). In a typical redox titration (e.g. FeSO4 with K₂Cr₂O₇), once all Fe²⁺ is converted to Fe3⁺, any excess dichromate will begin to oxidise diphenylamine, which changes colour — typically from colourless to a deep blue or violet, indicating that the endpoint has been reached.