The -electron approximation is a method for determining the energies of planar conjugated organic compounds by treating the
electrons separately from the
electrons.
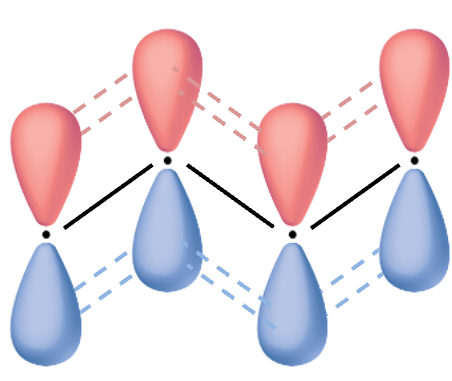
In this approximation, valence electrons in conjugated organic compounds are assumed to form the planar molecular framework. They are considered relatively unreactive for reactions that do not involve in breaking these
bonds.
valence electrons, on the other hand, participate in many reactions. They are perceived as moving in some fixed electrostatic potential due to the
valence electrons.
If we further assume that the total valence wavefunction of a planar conjugated organic compound has the separable form , we can express the Hamiltonian
as
where
It follows that the total energy of the valence electrons is
where and
.
As we have assumed that valence electrons are involved in reactions, we need only to solve for
. This is done using eq157 of the Hartree-Fock-Roothaan method, with
, where
is the antisymmetriser and
. The basis functions
are the
orbitals of the
carbon atoms of the planar molecule.
Evaluating for larger molecules using the aforementioned ab initio procedure may be challenging. To streamline the computation, we can introduce additional assumptions, which leads us to the Hückel method.

Question
What does ab initio mean?
Answer
Ab initio means “from the beginning” in Latin. An ab initio method involves deriving some parameters from first principles and not from experimental data. The Hartree self-consistent field method, the Hartree-Fock method and the Hartree-Fock-Roothaan method are all ab initio methods.