Thermodynamic functions involving the canonical partition function describe thermodynamic properties of a canonical ensemble.
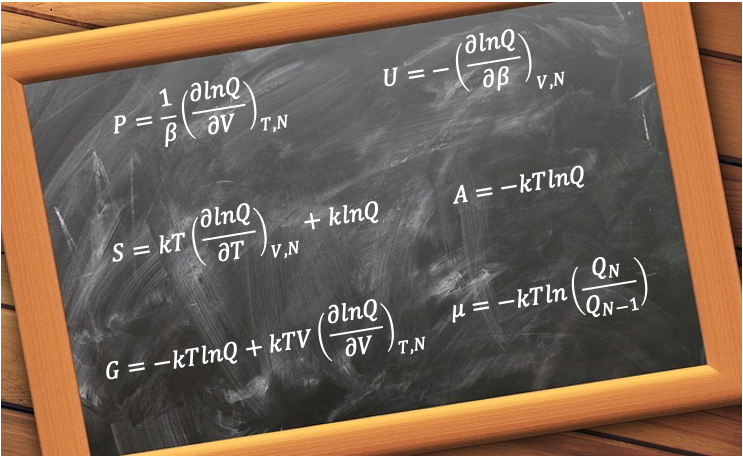
In statistical mechanics, the canonical ensemble provides a powerful framework for connecting microscopic states to macroscopic thermodynamic properties. Central to this approach is the canonical partition function, , which encapsulates the statistical behaviour of a system in thermal equilibrium at fixed temperature, volume, and particle number. From
, one can derive key thermodynamic functions such as pressure, internal energy and entropy through straightforward mathematical relations.
In the previous article, we derived the expressions for pressure and internal energy:
where and
.

Question
How does connect quantum mechanics to thermodynamics?
Answer
, the pressure of a system, is a macroscopic thermodynamic property, while
is the energy of a quantum mechanical microstate, which is an eigenvalue of the Hamiltonian. Therefore, the equation provides a bridge between the microscopic quantum description and macroscopic thermodynamic observables.
The corresponding expression for entropy can be derived by first dividing the fundamental equation of thermodynamics by
to give:
Applying the chain rule to eq148 gives:
Substituting eq146 and eq157 into eq156 yields:
Integrating eq158 results in , where
is a constant. As
, only the ground state
is populated. Assuming the ground state is non-degenerate, the partition function becomes
, with
. According to the third law of thermodynamics, entropy approaches zero as the temperature approaches absolute zero. Therefore,
must be zero to satisfy this requirement, resulting in:
It follows that the Helmholtz energy expression is:
Using the pressure and internal energy equations, the expression for enthalpy is:

Question
Does eq159b contradict the application of enthalpy, which describes a system at constant pressure?
Answer
The definition of enthalpy, , holds for any thermodynamic state, regardless of whether the pressure is constant. The condition of constant pressure becomes relevant when evaluating the change in enthalpy,
, which equals the heat exchanged in a constant-pressure process
. Eq159b expresses the enthalpy of a system in terms of
,
and
. It gives the enthalpy for a particular equilibrium state, not just under constant pressure. Therefore, we can calculate the enthalpy of a system at any given state using eq159b. By evaluating it at two different states (e.g. the initial and final states of a process), we obtain
. If the process connecting those states occur at constant pressure, then
. So, there is no contradiction.
The expression for constant-volume heat capacity is:
For Gibbs energy, we have . Hence,
If we define chemical potential as , and
is an integer-valued discrete variable, then the derivative becomes a finite difference, so: