Ultraviolet-visible (UV-Vis) spectroscopy is an analytical technique that measures the amount of discrete ultraviolet and visible light that is absorbed by a sample, allowing for the identification and quantification of various compounds.
A photon of UV-Vis radiation possesses an energy given by the equation
, where
represents the Planck constant,
denotes the speed of light and
signifies a wavelength in the range of 200 to 800nm. This quantum of energy corresponds to the energy difference between the highest occupied orbitals and lowest unoccupied orbitals in many atoms, ions and molecules. When these chemical species absorb UV-Vis photons, they undergo electronic transitions between these orbitals. As different chemical species absorb at characteristic wavelengths, they can be identified through UV-Vis spectroscopy.
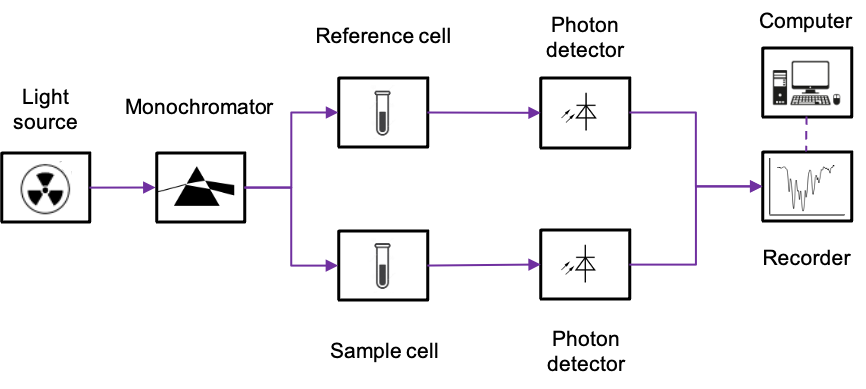
A typical double-beam UV-Vis spectrometer comprises a light source that emits a beam of broad-spectrum electromagnetic radiation with a wavelength between 200 and 800nm (see above diagram). Three light sources, namely the deuterium arc lamp, the tungsten-halogen lamp and the Xenon flash lamp, are commonly utilised. The generated light beam initially passes through a monochromator, where it is split into its component wavelengths. A beam with a very narrow range of wavelengths is then selected and divided into two using mirrors. These beams are separately directed towards the reference cell and the sample cell. For an aqueous sample, the reference cell is filled with the corresponding solvent. Otherwise, it remains empty.
The photodetector operates based on the photoelectric effect. This is where the interaction of UV-Vis radiation with a semiconductor promotes electrons to the conduction band, thereby generating a small current that is proportional to the intensity of the radiation. The magnitude of the current is separately recorded for sample cell and the reference cell, and the final spectrum is generated by the computer using a ratio of the sample spectrum against the reference spectrum.
In the visible electromagnetic range, UV-Vis spectroscopy proves useful in determining the concentration of coloured aqueous compounds. This is largely due to the Beer-Lambert law, which is applicable for most diluted aqueous compounds. For instance, if a 0.10M solution of copper(II) sulphate yields an absorbance of 0.55, the absorbance
when the concentration is doubled can be calculated as follows:
Beyond a single measurement, UV-Vis spectroscopy can also be employed to monitor changes in concentration of a coloured compound during a reaction.
In the realm of organic chemistry, it is possible to predict the extent of UV-Vis absorption by organic compounds using the principles of UV-Vis spectroscopy. For example, a saturated organic compound is likely to only absorb short wavelengths of UV light, while a conjugated compound and an aromatic compound may absorb light with wavelengths in the visible range.