The Zeeman effect is the splitting of atomic or molecular spectral lines into multiple components when the emitting or absorbing species is placed in an external static magnetic field.
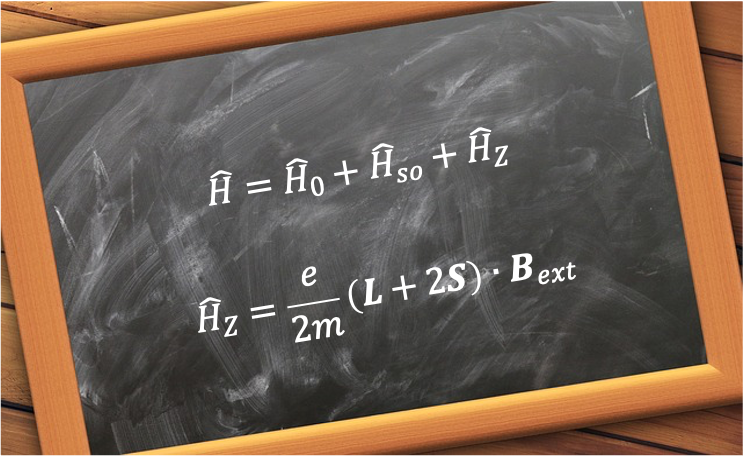
The extent of the splitting depends on the strength of the external field relative to the internally generated magnetic field
arising from spin-orbit coupling, with the Hamiltonian
of the system generally expressed as:
where is the non-relativistic Hamiltonian, and
and
are the perturbations due to spin-orbit coupling and the Zeeman effect respectively.
Consider a one-electron system, such as the hydrogen atom. The Zeeman Hamiltonian is given by the quantum mechanical analogue of eq62:
where, from eq61 and eq164, and
respectively.
To fully understand the Zeeman effect, we must analyse how external magnetic fields of different magnitudes (broadly classified as the weak-field, intermediate-field and strong-field regimes) influence the splitting of degenerate energy levels in the hydrogen atom.