What is the formula of the titration curve of a monoprotic weak acid versus a monoprotic weak base?
With reference to previous articles, substitute eq6, eq10, Kw = [H+][OH–] and [H+] = 10-pH into eq1 (where Ca and Cb are now the concentration of a weak acid and the concentration of a weak base respectively), we have,
Eq12 is the complete pH titration curve for a monoprotic weak acid versus monoprotic weak base system. We can input it in a mathematical software to generate a curve of pH against Vb. For example, if we titrate 10 cm3 of 0.200 M of CH3COOH (Ka = 1.75 x 10-5) with 0.100 M of aqueous NH3 (Kb = 1.8 x 10-5), we have the following:

To understand the change in pH near the stoichiometric point versus the change in Vb, we assume that one drop of base is about 0.05 cm3 and substitute 19.95 cm3, 20.00 cm3 and 20.05 cm3 into eq12. The respective pH just before and just after the stoichiometric point (SP) are:
|
|
One drop before SP | SP |
One drop after SP |
|
Volume, cm3 |
19.95 | 20.00 |
20.05 |
|
pH |
6.91 | 7.01 |
7.10 |
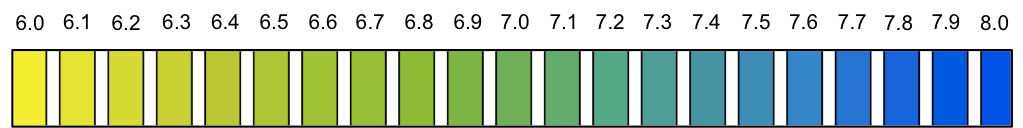
The data shows that two drops of base cause a change of only 0.19 in pH before and after the stoichiometric point. Since the change in pH at the stoichiometric point occurs within a very narrow range, no indicator is suitable to accurately monitor the stoichiometric point, not even bromothymol blue (see diagram below for bromothymol blue colour at various pH).
Lastly, we can derive the gradient equation for a weak acid to weak base titration and investigate the inflexion point at pH 7.01 by differentiating eq12 implicitly (see this article), resulting in , i.e. a gradient that makes angle of 62.71o with the horizontal.