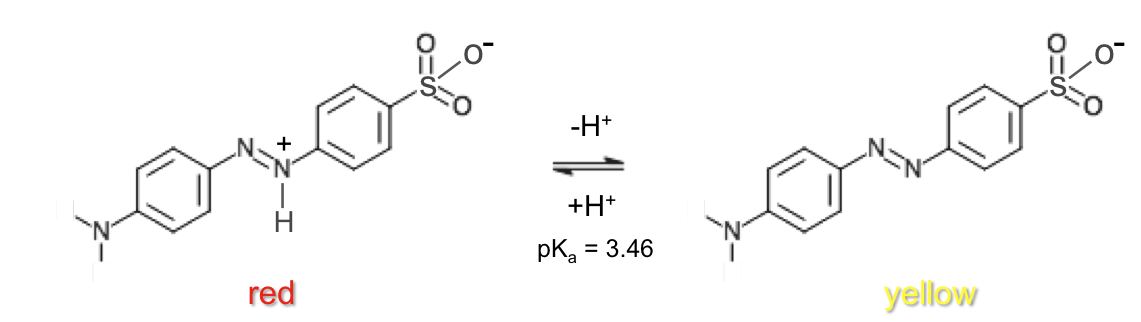
Methyl orange is a commonly used indicator. Protonation of the nitrogen at the para position of the benzenesulphonate moiety is preferred over the negatively charged oxygen of the sulphonate group at low pH, as that will confer resonance stabilisation (benzenoid-quinonoid tautomerism) to the acid form of methyl orange.
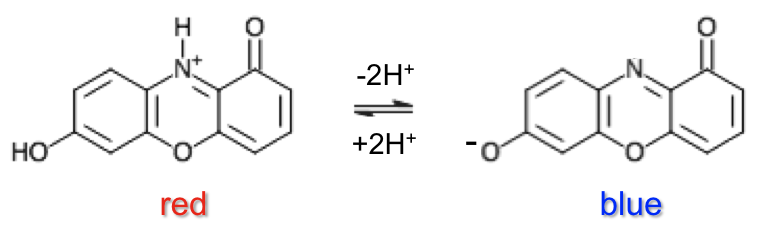
Litmus is extracted from lichen, processed and applied onto filter paper. It has a pH range of 4.5 to 8.3 and contains the chromophore, 7-hydroxyphenoxazone, which occurs in the following states:
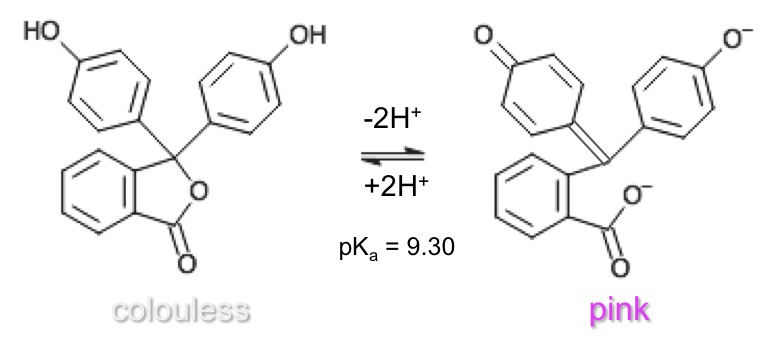
Phenolphthalein has three pKa values with four different forms. However, stoichiometric points of titrations are most commonly monitored with the middle pKa value of 9.30, which involves the lactone and phenolate (dianionic) forms:
Thymol blue has two transition range with pKa1 = 1.65 and pKa2 = 8.96.

