The reactivity of metals broadly refers to the relative extent of change that metals undergo with respect to certain reactions. Some of these reactions are:
In general, the greater the extent of change of a metal from its elemental form to a compound is, the more reactive it is. For example, sodium is more reactive than copper, as elemental sodium reacts violently with cold water, while elemental copper does not react with water and steam. The reactivities of metals for reactions 1 to 5 are summarised in the following table that is known as the metal reactivity series:
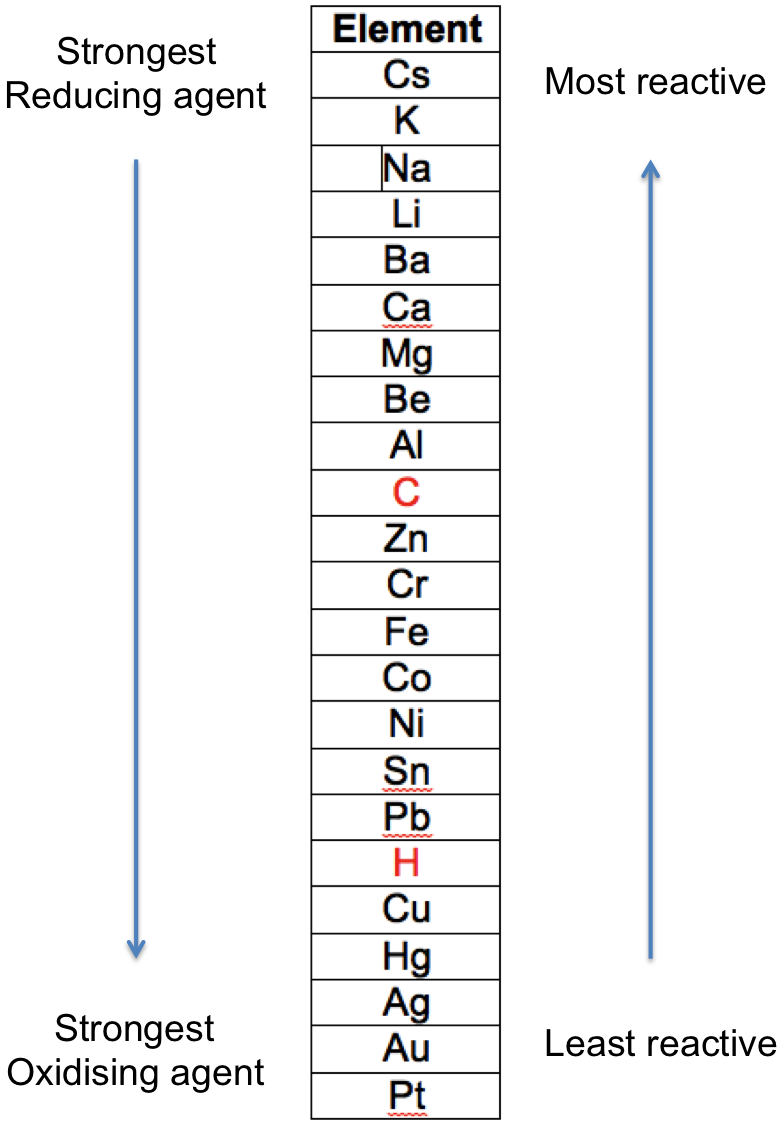
The two non-metals, C and H are included for reference.
