There are many ways to measure the rate of a reaction, some of which are depicted in the diagram below.

The rate of a reaction that produces a gas can be monitored by measuring the volume of gas produced at different time with a syringe, or by noting the pressure of the gas at various time intervals with a pressure gauge (A). Another way to determine the rate of a reaction is to measure the change in mass of a reaction mixture over time (B). We can also measure the rate of a reaction via colorimetry (C) if a reacting species is coloured, and by titrimetric methods (D) if a reacting species can be oxidised or reduced by a titrant.
The rate of reaction between hydrochloric acid and calcium carbonate to give carbon dioxide is an example that can be determined by either method A or B.
With respect to the syringe method A, the volumes of CO2 recorded are plotted against time (see diagram below), where the gradient at any point on the curve is the change of the amount of product versus the change in time, i.e. the rate of the reaction given by eq1.
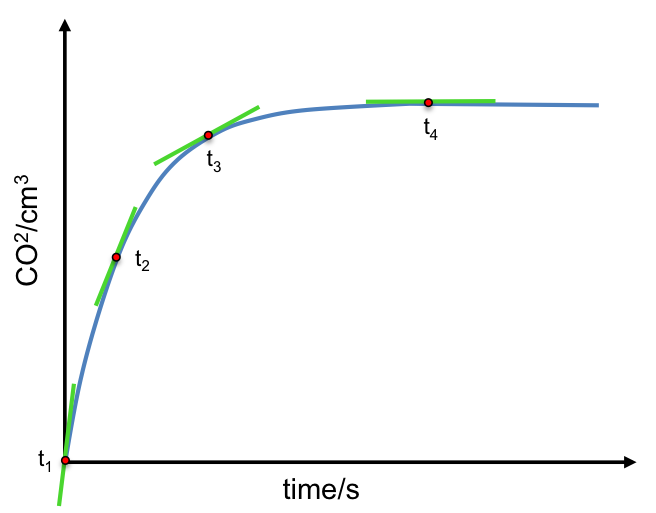
The graph shows that the gradient is the steepest at the beginning of the reaction, t1, which means that the rate of reaction is fastest at the beginning. As the reaction progresses, the gradient decreases at t2 and t3, implying that the rate of reaction is decreasing. This is due to decreasing concentration of reactants, which are converted to products over time. Finally at t4, the gradient is zero, signifying that the reaction has stopped.
The above rates that are measured at specific times of the reaction are called instantaneous rates of reaction. We can also measure the average rate of reaction over a certain period of time just like we measure the average speed of a car over a certain stretch of a journey. For example, the average rate of reaction for the production of CO2 over the 1st five minutes of the reaction is:
where is the volume of CO2 at x seconds.
Since a reaction’s rate generally decreases over time, we need a way to compare the rates of reaction between different reactions. This can be done using the initial rate of reaction, which happens to be a very useful analytical tool in chemical kinetics. Conceptually, the initial rate of reaction is the rate of reaction just after the start of the reaction when t ≈ 0, i.e.we measure the initial rate of reaction immediately after the beginning of the reaction. As elaborated in subsequent sections, this is a good reference point to compare the rates of reaction of reactants with different concentrations because their concentrations are assumed to be approximately the same as that before the start of their respective reactions.