Entropy, denoted by the letter S, is a measure of energy dispersal at a specific temperature.
From the articles on chemical energetics, we learn that a reaction’s enthalpy change indicates whether it is exothermic or endothermic. Scientists previously thought that only exothermic reactions occur spontaneously, as the energy of systems are lowered during such reactions in a way similar to the lowering of an object’s gravitational potential energy when it falls from a height to the ground. However, the change in enthalpy of a reaction does not predict whether the reaction is spontaneous, because both exothermic and endothermic reactions can occur spontaneously. For example, both NaCl and NaOH dissolve spontaneously in water but the enthalpy change of solution of NaCl is positive, while that of NaOH is negative.
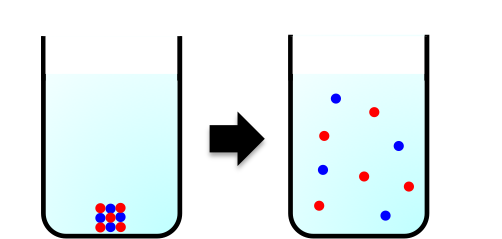
One common aspect of the solution of NaCl and the solution of NaOH is the change of the ions from an ordered solid lattice structure to a random distribution of solvated ions throughout the vessel (see diagram above). Similarly, when ice dissolves spontaneously to form water at a temperature above 0°C, the regular arrangement of H2O molecules in ice becomes a disordered distribution of H2O molecules.
This suggests that chemical reactions occur spontaneously in a direction that increases their energy dispersal, which is sometimes inaccurately termed as ‘disorderliness‘. To quantify the change in the energy dispersal of a system, a measure called entropy is developed.