Hund’s rule states that electrons occupy orbitals of a sub-shell singly and in parallel before pairing up (see diagram below for the n = 2 shell of carbon). This is sometimes called the ‘bus seat’ rule, where bus passengers who travel alone tend to occupy empty double-seats before partly occupied seats.
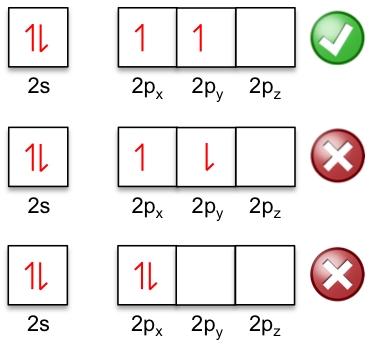
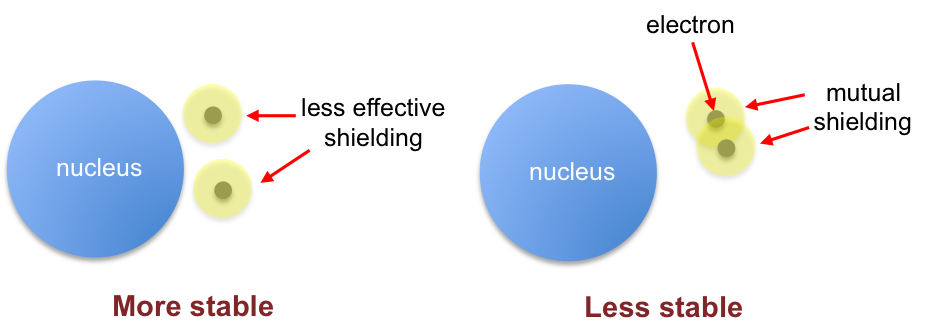
By spacing themselves apart in different orbitals, electrons attain a relatively stable configuration. This is because electrons offer one another less shielding from the nuclear charge if they are further apart. The decreased shielding leads to the electrons being stabilised closer to the nucleus, resulting in a lower energy system (see digram below).
But why do the electrons that occupy the p-orbitals singly in the above example have parallel spins? A crude explanation is to visualise an electron with a north pole and a south pole (a dipole) due to the magnetic field that it generates with its spin. To stay far apart from each other to reduce repulsion, the dipoles of the two electrons in the p sub-shell must be aligned in a parallel fashion. If the dipoles are anti-parallel, the electrons will be closer to each other, resulting in a higher and less stable energy state. The proper explanation for Hund’s rule, however, lies in the symmetry of the wave function of the system.

Question
Why can’t the n = 2 electron configuration of carbon be 2s12px12py12pz1?
Answer
The stability of the ground state of carbon is a result of the interplay between Hund’s rule and the Aufbau principle. Since the 2s orbital is of a lower energy than each of the 2p orbitals, the electron configuration of the n = 2 shell of 2s22px12py12pz0 offers greater stability to the system than the configuration of 2s12px12py12pz1.
To understand why the ground state electron configuration of chromium is [Ar]3d54s1 instead of [Ar]3d44s2 and that of copper is [Ar]3d104s1 instead of [Ar]3d94s2, we need to consider the following:-
-
- The 3d sub-shell has a lower energy than the 4s sub-shell for Z > 20 (see previous article).
- Electrons occupying 3d orbitals experience greater repulsions than electrons residing in 4s orbitals (see previous article).
- Hund’s rule states that electrons prefer to stay apart from one another by occupying orbitals singly before pairing up.
The electronic configuration of a 1st row transition metal is dependent on the interplay of the above three factors. For chromium, the energy level of the 3d sub-shell is very close to that of the 4s sub-shell and hence the 3rd factor outweighs the 2nd one. For copper, the energy level of the 3d sub-shell is much lower than that of the 4s sub-shell such that the 1st factor outweighs the other two factors.
