The earlier models of the atom were constructed using classical mechanics. When Niels Bohr introduced his model of the atom, he not only utilised Newtonian mechanics in his derivation but also incorporated the Planck relation E = hv, which was conceived a decade earlier by Max Planck, a German physicist.
In 1900, Planck was trying to develop a formula to describe the radiation spectrum of a black body when he suggested that electromagnetic radiation is a form of energy that is quantised. The significance of this concept eventually led to development of quantum theory, with Planck being regarded as the father of quantum mechanics.
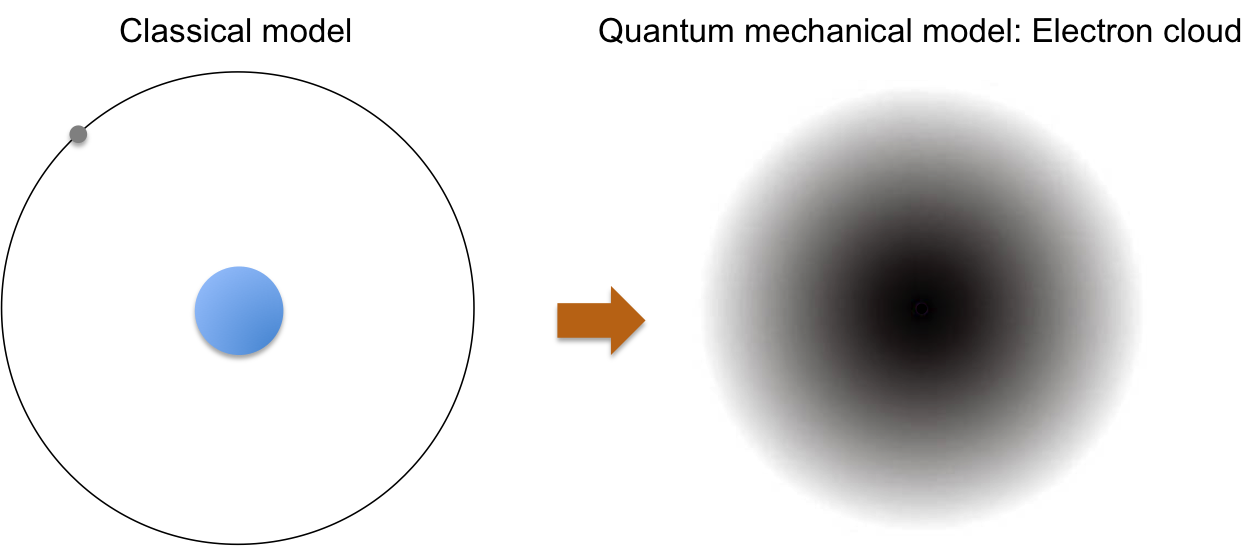
Quantum mechanics is key to the elucidation of the modern structure of an atom, where electrons are no longer perceived as orbiting in defined paths around the nucleus. Instead, an atom is represented by equations that describe the probability distribution of electrons in space, giving rise to a nucleus that is surrounded by an electron cloud (see below diagram).
In the modern interpretation of the atomic structure, electrons are distributed in an atom in specific energy states that are characterised by mathematical functions known as orbitals. The maximum number of electrons that an orbital can accommodate is two (see this article for details). Orbitals with similar shapes form a sub-shell (characterised by a unique set of (n, l), e.g. px, py and pz forms the sub-shell p), and sub-shells with the same energy in the absence of an external magnetic field, constitute a shell (e.g. 2s and 2p sub-shells constitute the shell n = 2). Diagrammatically, we can describe the energy states as follows:
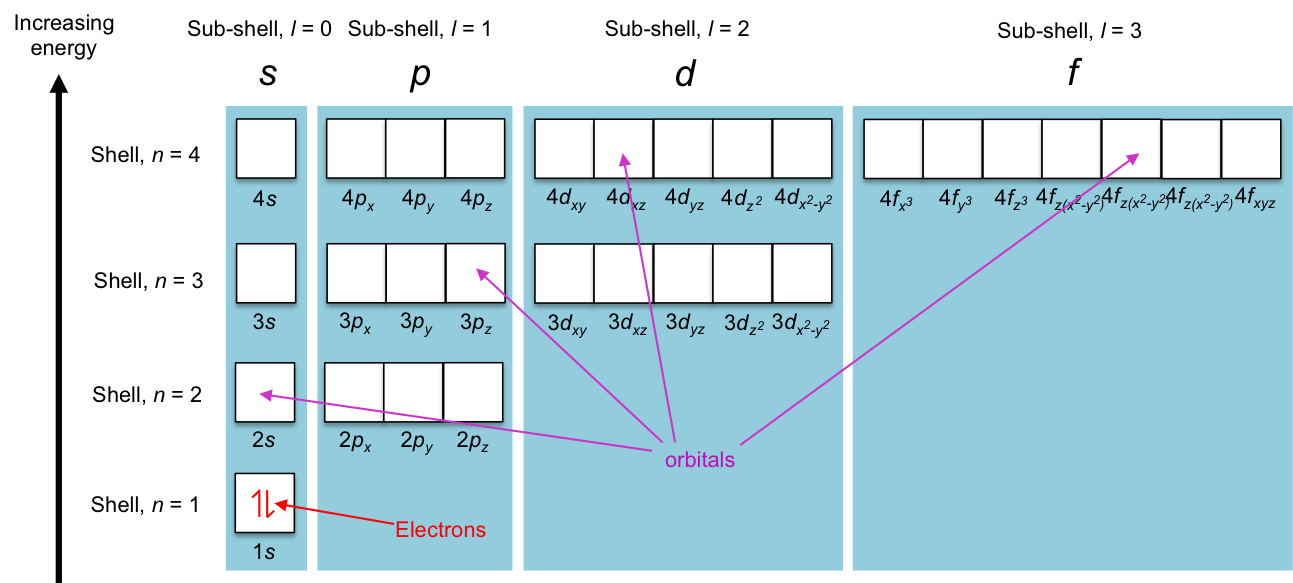
Mathematically, the energy states are defined by four quantum numbers, n, ,
and ms, as shown in the table below.
|
Quantum numbers |
Details |
Example |
||
|
Symbol |
Name |
Values |
||
| n | Principal | Each value of n refers to a shell |
n = 1 and n = 2 are the 1st shell and 2nd shell of an atom respectively. |
|
| Angular | Each value of |
For the 1st shell (n = 1), For the 2nd shell (n = 2), |
||
| Magnetic | with a total of |
Each value of |
For the 1st shell (n = 1), For the s sub-shell in the 2nd shell, For the p sub-shell in the 2nd shell, |
|
| Spin magnetic | Each value of |
|
||
In other words, the four quantum numbers describe the energy state of an electron in an atom. The numbers are a result of many scientists’ work that were done during the early 1900s. Some of these experiments and theories that contributed to the development of quantum mechanics are listed in the table below.
|
Year |
Work | Scientist |
|
1900 |
Planck’s law |
Max Planck |
|
1905 |
Photoelectric effect |
Albert Einstein |
|
1924 |
de Broglie’s hypothesis |
Louis de Broglie |
|
1925 |
Schrodinger equation |
Erwin Schrodinger |
|
1925 |
Pauli exclusion principle |
Wolfgang Pauli |
|
1926 |
Born interpretation |
Max Born |
| 1920-1930 | Aufbau principle, Madelung’s rule and Hund’s rule |
Niels Bohr, Wolfgang Pauli, Erwin Madelung, Friedrich Hund |
|
1927 |
Heisenberg’s uncertainty principle |
Werner Heisenberg |
We shall elaborate on the above in the following articles.

Question
Does the energy level diagram for shells and sub-shells apply to all atoms?
Answer
The above energy level diagram is a result of the solution of the Schrodinger equation for the hydrogen atom, which has degenerate sub-shells, i.e. sub-shells belonging to a particular shell (e.g. 2s and 2p for n = 2) have the same level of energy. The degeneracy of sub-shells disappears for multi-electron atoms due to electron-electron repulsion and the shielding effect of orbitals. For a particular shell in a multi-electron atom, the smaller the angular quantum number, the lower the energy level of the sub-shell within that shell.
