The Davisson-Germer experiment studies the scattering of electrons by a nickel single crystal. In 1927, Clinton Davisson and Lester Germer, both American physicists, irradiated a nickel single crystal with a 54 eV electron beam* and rotated the detector at various angles to capture the scattered electrons (see below diagram).
* The electron beam energy of 54 eV or 8.65 x 10-18 J is attributed to the kinetic energy of an electron (see this article for details).
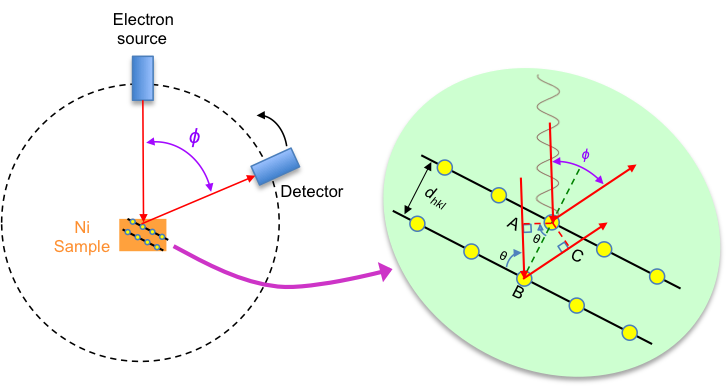
Prior to the experiment, an electron is known to be a particle with a mass of 9.1 x 10-31 kg. In 1924, de Broglie hypothesised that electrons also possess wave characteristics. If so, and if electrons have wavelengths similar to the interatomic distance (or interplannar distance) of nickel in the experiment, they would be diffracted by the nickel crystal, resulting in interference patterns typically seen in Thomas Young’s diffraction experiments.

The results indeed produced such a diffraction pattern, with a first-order constructive interference peak at φ = 50° (see diagram above). According to Bragg’s law,
where d is the interplannar distance and n is the order of diffraction.
With reference to the top diagram, . Substituting
, the interplannar distance of Ni of 91 pm and n = 1 in eq5, the wavelength of the electron is λ = 165 pm.
To test de Broglie’s hypothesis, we rearrange de Broglie’s relation as follows:
Substituting the value of the Planck constant (6.62 x 10-34 m2 kg s-1), the mass of an electron (9.1 x 10-31 kg) and kinetic energy of an electron (54 eV = 8.65 x 10-18 J ) in eq6, we get λ = 167 pm, which is in very close agreement with the experimental value of 165 pm.
The Davisson-Germer experiment therefore verifies the wave characteristic of electrons and de Broglie’s hypothesis.
Subsequently, other experiments involving other elementary particles, atoms and even macromolecules like C60 were conducted, all of which, validated de Broglie’s hypothesis. The technique employed by Davisson and Germer is now known as low energy electron diffraction (LEED), which is utilised to study surface properties of material.
