A Slater determinant represents a multi-electron wavefunction that satisfies the Pauli exclusion principle. It was named after the American physicist John Slater for his contribution to quantum mechanics.
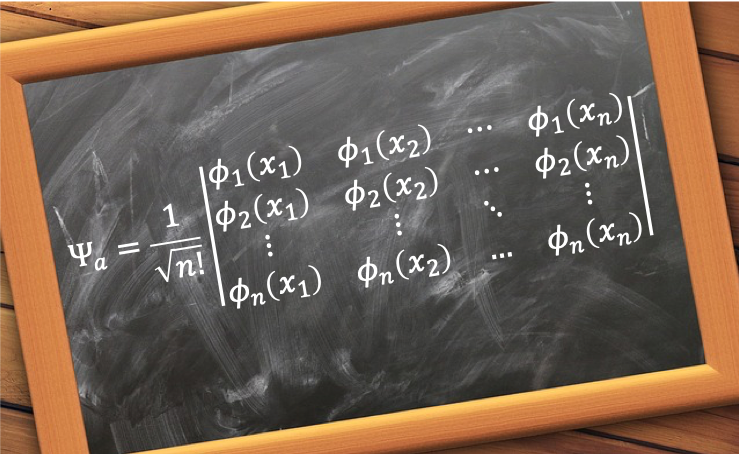
The normalised anti-symmetric wavefunction for an n-electron system with spin-orbitals can be expressed as
, where
and
is the antisymmetriser. From eq59 and 62,
Comparing the above equation with the definition of a determinant, i.e. , we have
or
which is called an n-electron Slater determinant.
Since (see this article for proof), the Slater determinant can also be written as
