The Carnot cycle, developed by the French scientist Sadi Carnot in 1824, is a theoretical construct that sets the maximum efficiency of a thermodynamic engine.
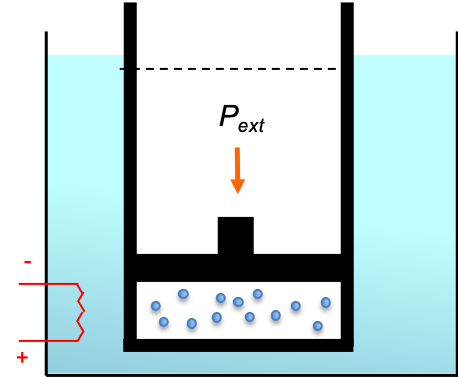
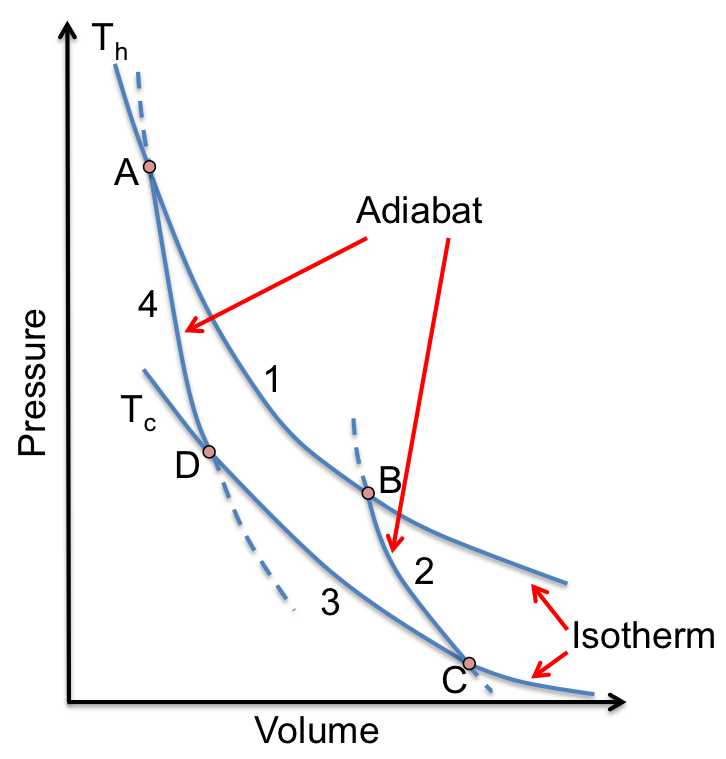
Consider a gas in a piston-cylinder device (see diagram above) that repeatedly undergoes a cycle consisting of four reversible processes as follows:
- (A to B) The pressure and temperature of the gas at A is equal to
and
respectively. At this point, the device is in thermal contact with a hot reservoir at a constant temperature
. Heat is transferred from the hot reservoir to the gas with
being reduced infinitesimally, causing the gas to expand and push the piston up to do work on the surroundings. This process is a reversible isothermal expansion of the gas.
- (B to C) The contact with the hot reservoir is removed and the device is thermally insulated.
is again allowed to decrease infinitesimally and the gas continues to expand to do work on the surroundings with its internal energy, resulting in a drop in temperature to
. This process is a reversible adiabatic expansion of the gas.
- (C to D) The device is placed in contact with a cold reservoir at a constant temperature
and the insulation is removed. Work is now done on the gas with
increased infinitesimally and heat is transferred from the gas to the reservoir. This process is a reversible isothermal compression of the gas.
- (D to A) The contact with the cold reservoir is removed and the device is thermally insulated again. Work done on the gas continues with
increasing infinitesimally, resulting in an increase in its internal energy and a hence an increase in temperature back to
. This process is a reversible adiabatic compression of the gas.
The theoretical piston-cylinder device that operates via the Carnot cycle is called a Carnot heat engine, i.e. one that operates by transferring energy from a body at a higher constant temperature to a body at a lower constant temperature and, through a cyclic process, converting some of that energy to mechanical work (see diagram below).
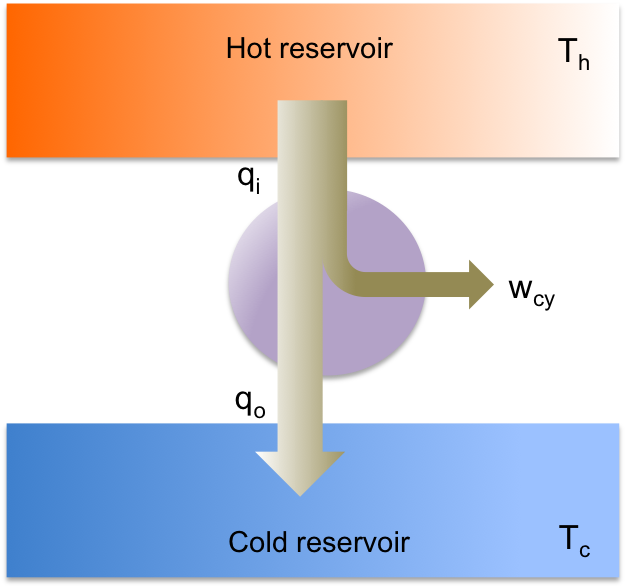
By the law on conservation of energy:
where is the transfer of heat into the system from the hot reservoir (
),
is the net work produced by the cycle (
) and
is the transfer of thermal energy out of the system into the cold reservoir (
).
The efficiency, , of a heat engine is defined as the fraction of heat transferred to the system from the hot body that is converted to work:
The modulus sign ensures that the value of is positive. Substituting eq95 in eq96,
The greater the work produced by the engine, the smaller the value of and hence the closer the efficiency of the engine to one.
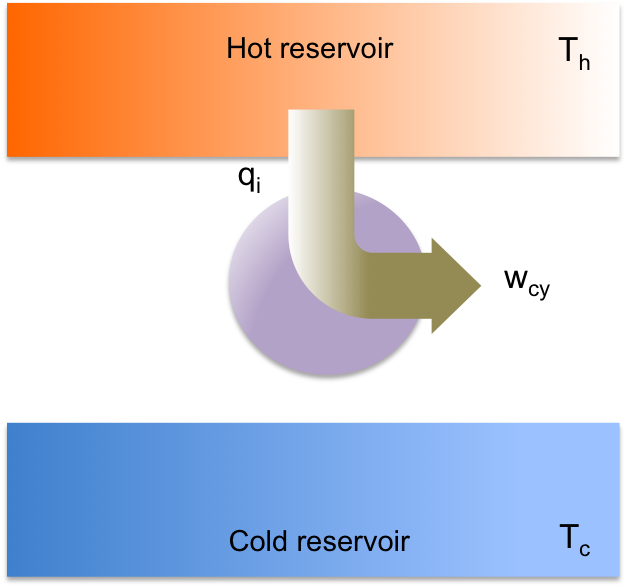
For a reversible heat engine to function, it can operate via different processes (e.g. reversible isochoric processes in place of reversible adiabatic processes). However, regardless of the processes involved, it must undergo a cycle where thermal energy is transferred from a hot reservoir to a cold reservoir with some of the energy converted to work, which is how a heat engine is defined. Therefore, it is impossible to construct an engine that undergoes a cyclic process where thermal energy extracted from the hot reservoir is completely converted to work without any energy deposited at the cold reservoir (see diagram above). This is the Kelvin-Planck statement of the second law of thermodynamics.

Question
Can an engine cycle consist of just the first and third processes i.e. a reversible isothermal expansion followed by a reversible isothermal compression?
Answer
If so, the net work done by the cycle is zero (net area under the PV curve is zero), which is not much of an engine.
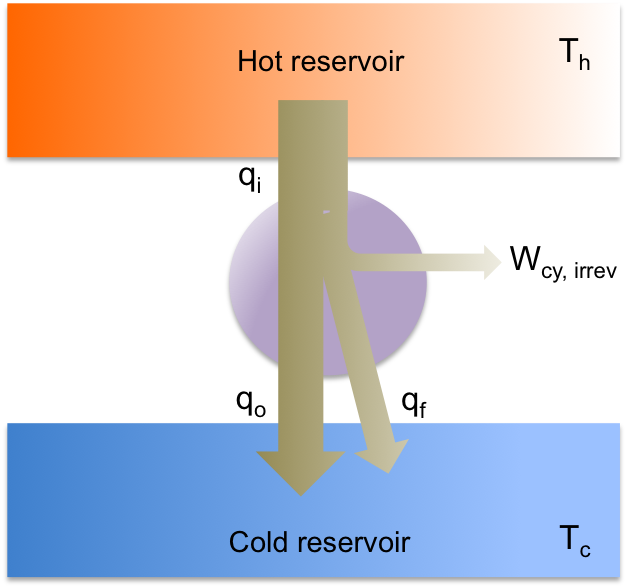
An irreversible heat engine (real heat engine) is less efficient than a Carnot engine due to the occurrence of dissipative processes during the cycle, e.g. friction. Some of the work is converted to heat, which is transferred to the cold reservoir as (see diagram above). So,
and according to eq96,
Even though we have proven that an irreversible heat engine is less efficient than a Carnot engine, we cannot conclude that the Carnot cycle sets the maximum efficiency of a thermodynamic engine. To do so, we need to understand the Carnot heat pump and Carnot’s theorem.

Question
For an ideal gas,
Show that eq98 is applicable to any reversible cycle for a system containing any substance.
Answer
Let’s assume that Carnot cycles of different working substances may have different shapes. Therefore, we need to prove that applies to any cycle.
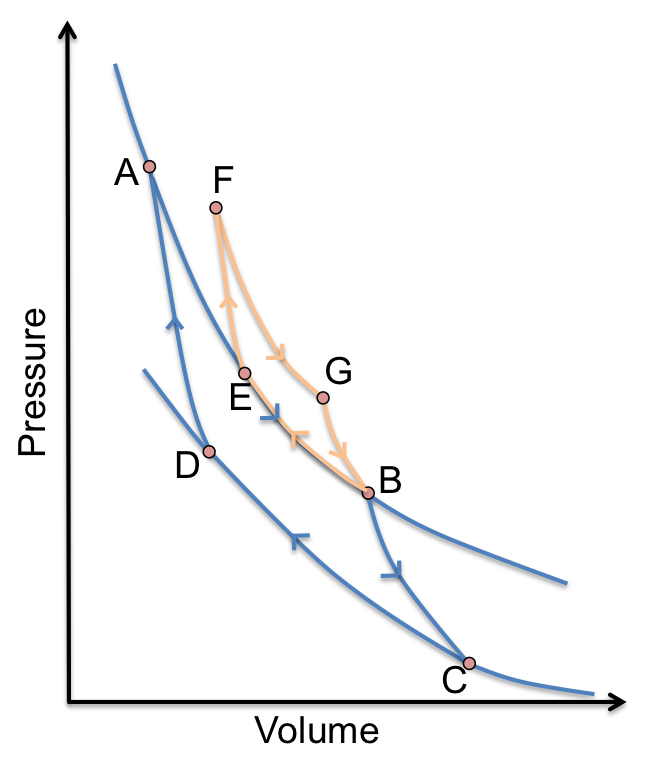
Consider a cyclic process AEFGBCDA (see diagram above). for AEFGBCDA is:
This cycle is actually made up of two Carnot cycles ABCDA and FGBEF with for ABCDA given by
and for FGBEF being:
Since and
,
Substitute eq102 in eq100
Adding eq101 and eq103 and comparing with eq99,
From eq98, and
. Therefore,
. This means that eq98 holds true for a cycle that is composed of two different sized Carnot cycles. The same logic can be applied to cycles that are made up of more than two Carnot cycles.
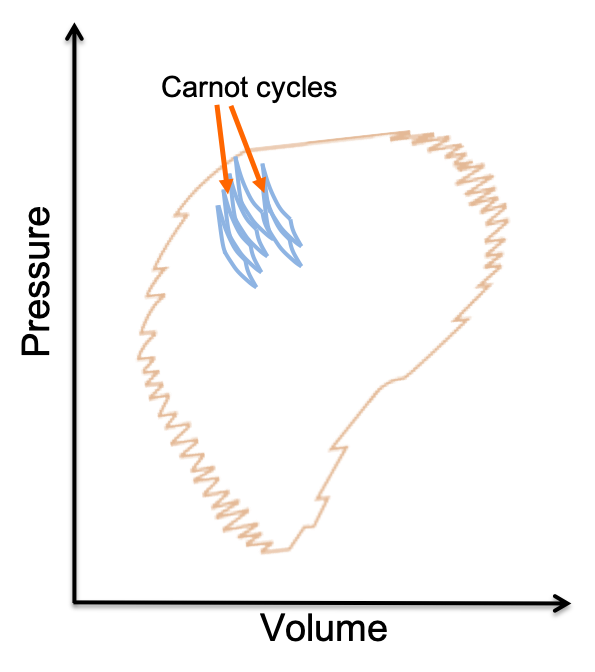
Let’s now look at an arbitrary reversible cycle that can represent a system containing any substance (see diagram above). The arbitrary cycle is exactly composed of an infinite number of Carnot cycles as the sizes of the Carnot cycles approach zero. Hence, for any arbitrary reversible cycle,
Therefore, eq98 applies to any reversible cycle for a system containing any substance.
