A reversible isobaric process is a reversible thermodynamic process that occurs at constant pressure.

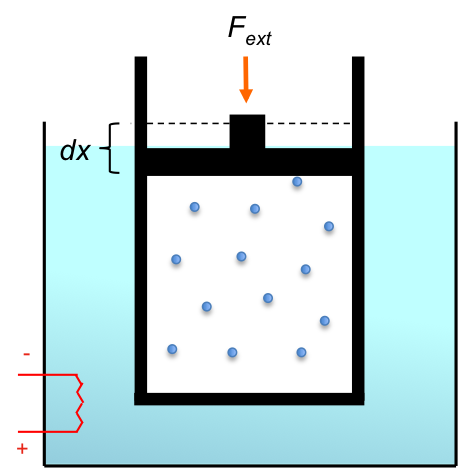
Specifically, a reversible isobaric process that follows the path of either A to B or D to C is a reversible isobaric expansion, while one that goes from either B to A or C to D is a reversible isobaric compression. We shall use the same setup of a piston-cylinder device immersed in a water bath as the one described in the previous article to illustrate reversible isobaric processes.
With reference to the ideal gas law, an isobaric expansion of a gas requires the ratio of to be constant as the volume of the system increases. Theoretically, for a reversible isobaric expansion to occur, the system has to be in contact with an infinite sequence of temperature baths, with one bath having a temperature that is infinitesimally higher than the next one. In practice, to achieve a result that is close to reversibility, the temperature of the water bath is continuously increased by infinitesimal amounts to maintain a temperature gradient across the cylinder walls so as to supply the system with energy for the expansion at constant pressure.
Since the pressure of the system remains constant and equals to that of the external pressure throughout the process, eq5 becomes
For a reversible isobaric compression, the temperature of the water bath is continuously decreased by infinitesimal amounts to maintain a constant pressure in the system. Many chemical reactions that involve phase changes are conducted under isobaric conditions, e.g. .

Question
The change in work done is sometimes represented by . How does this relate to eq6 and eq7?
Answer
The expression or by convention
is the general differential equation for work. If the change in pressure is infinitesimal,
, then the expression reduces to
. If the process is a reversible isothermal process, we substitute the ideal gas law in
to give
, which is the differential form of eq6. If the process is isobaric,
, and
again reduces to
, which is the differential form of eq7.