Vibrational Raman selection rules describe the allowed changes in a molecule’s vibrational states during inelastic light scattering.
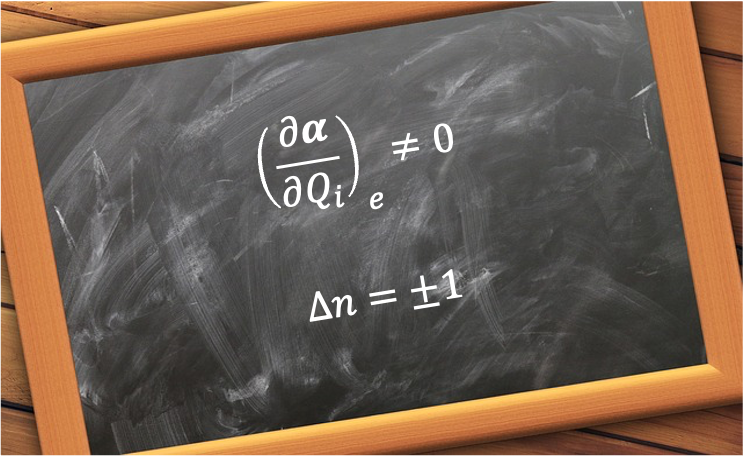
According to the time-dependent perturbation theory, the transition probability between the orthogonal vibrational states
and
is proportional to the square of the corresponding transition matrix element
. For example, in conventional infrared vibrational spectroscopy, this involves the electric dipole moment operator
, and transitions occur only for molecules with a permanent dipole moment.
Transitions associated with scattering radiation, however, arise from an induced dipole moment rather than a permanent one. It follows, with reference to eq1, that the transition probability is given by:
Thus, a necessary condition for scattered-radiation transitions is that the matrix element be non-zero. To examine this condition further, we consider how the molecular polarisability
depends on the normal coordinate
for a molecule. This dependence can be expressed through a Taylor series about the equilibrium position:
For the sole excitation of a single normal mode , we multiply eq40 on the left and right by
and
respectively and integrate over all space to give,
where the second equality is due to the fact that for all the other non-excited normal modes, which remain at their equilibrium positions.
Because the wavefunctions are orthogonal, the first term vanishes unless , which corresponds to Rayleigh scattering. Consequently, a Raman transition can occur only if
, i.e. the polarisability of the molecule must change as it undergoes vibrational motion.
Setting and expanding eq41 yields:
Substituting the recurrence relation eq32ab in eq42 gives:
For to be non-zero,
or
. Therefore, the vibrational Raman selection rules are