pH, which stands for power of hydrogen, is a measure of the acidity of an aqueous solution. It is defined as:
where [H+] is the concentration of the aqueous hydrogen ion.
The concept was developed by the Danish chemist, Soren Peder Lauritz Sorensen in 1909.


Question
Why is pH defined on a logarithmic scale and why does it have a negative sign?
Answer
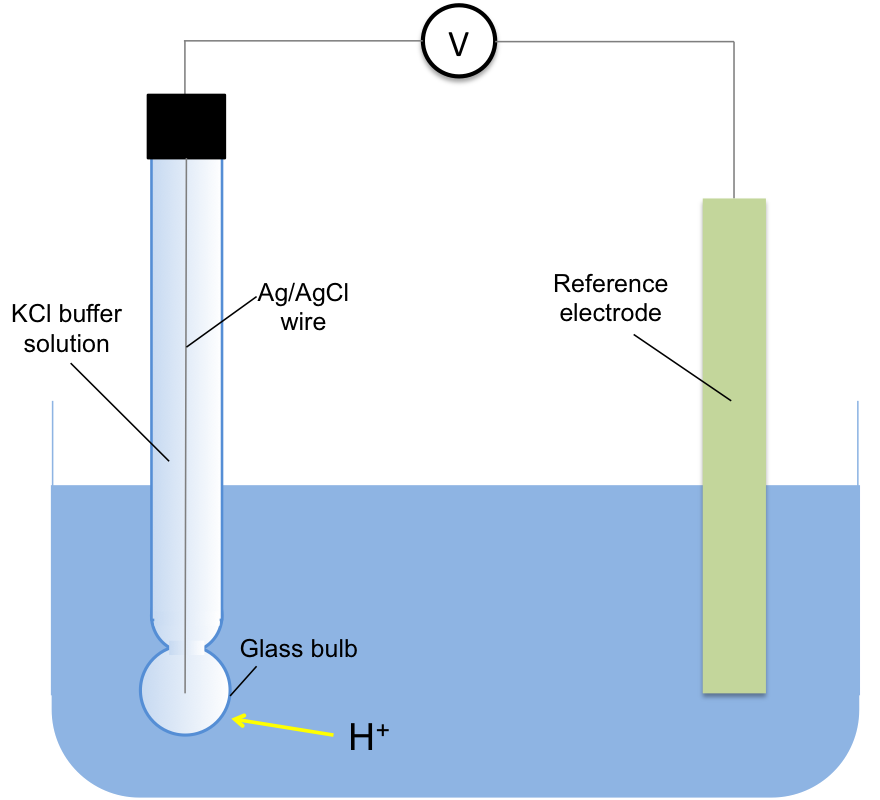
The most accurate way of measuring the concentration of H+ in a solution is using a glass electrode, a type of ion-selective electrode (see below diagram). The voltage, E, of the electrochemical cell containing the electrode is measured at 25oC and the concentration of H+ is determined by the Nernst equation:
Scientists found that the values generated by the factor log[H+] at different voltages are accurate over the range of -14 to 0 and decided to use it to conveniently express the concentration of H+ in a solution. As it is easier to deal with positive numbers (i.e. 0 to 14 instead of -14 to 0), pH is defined as –log[H+], which makes eq2:
However, there is more to Eq1. The formal definition of pH is actually
where is the activity of the hydrogen ion.
Activity is a measure of the “effective concentration” of a species in a mixture. It reflects how the behaviour of a species deviates from ideal behaviour due to interactions with other components. In an ideal solution, ions behave independently. However, in a real ionic solution, ions interact with each other — especially oppositely charged ions that loosely cluster together. These interactions make an ion less available to participate in chemical or electrochemical processes. So even if the actual concentration of an ion is high, its effective concentration (activity) is lower. Activity also applies to neutral molecules in liquids and to gaseous molecules.
In general,
where is the concentration of the
-th species in its standard state, which is defined as 1 mol dm-3, and
is the activity coefficient, where
for an ideal solution and
for a non-ideal solution.
How then is eq1 equivalent to eq2a? The pH formula (eq1) we commonly use assumes a dilute solution, which approaches ideality because the solute particles are far apart, resulting in negligible interactions between them. Combining eq 1 and eq2b gives:
Since the standard state of a solute is defined as 1 mol dm-3, we get . Here,
is unitless, as the units mol dm-3 cancel out in the ratio
. This is consistent with the mathematical requirement that the argument of a logarithm must be a pure number (i.e. dimensionless).
