An electric double layer consists of two layers of opposite charges that accumulate at the phase boundary between an electrode (a solid electrical conductor, e.g. a piece of zinc) and an electrolyte (a molten or an aqueous electrical conductor, e.g. aqueous zinc sulphate).

Zinc, according to the metal reactivity series, is a relatively reactive metal. When a zinc rod (an electrode) is placed in aqueous zinc sulphate, zinc atoms are easily oxidised to zinc ions, which enter the solution leaving free electrons on the surface of the electrode. At the same time, some zinc ions in the solution combine with free electrons in the electrode and are reduced to zinc atoms. Initially, more zinc atoms are oxidised to zinc ions than zinc ions are reduced to zinc atoms. As the concentration of zinc ions in the solution increases, an equilibrium is attained such that the rate of oxidation equals to the rate of reduction:
The equilibrium position for zinc, when compared to less reactive metals, lies to the left of the above equation, resulting in an excess of electrons on the surface of the electrode, which attract zinc ions in the solution that are close to the surface to form two parallel array of charges called an electric double layer. Just like a ball at a certain height has the capacity to do work, opposite charges that are separated at a distance have the potential to do work too. We call such a potential, which arises due to the formation of the electric double layer, an electrode potential.
As the formation of the ions and electrons in the electric double layer depends on the reactivity of the metal, the electrode potential of a Zn/ZnSO4 system is greater than that of a less reactive metal, such as copper, which is characterised by an equilibrium position in aqueous copper sulphate that lies to the right of the following equation:
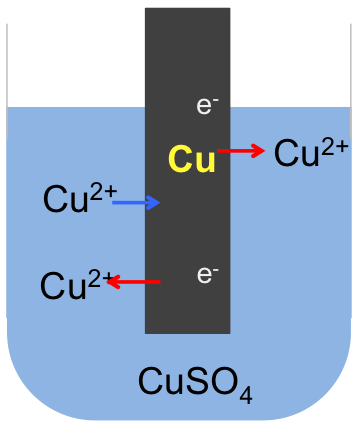
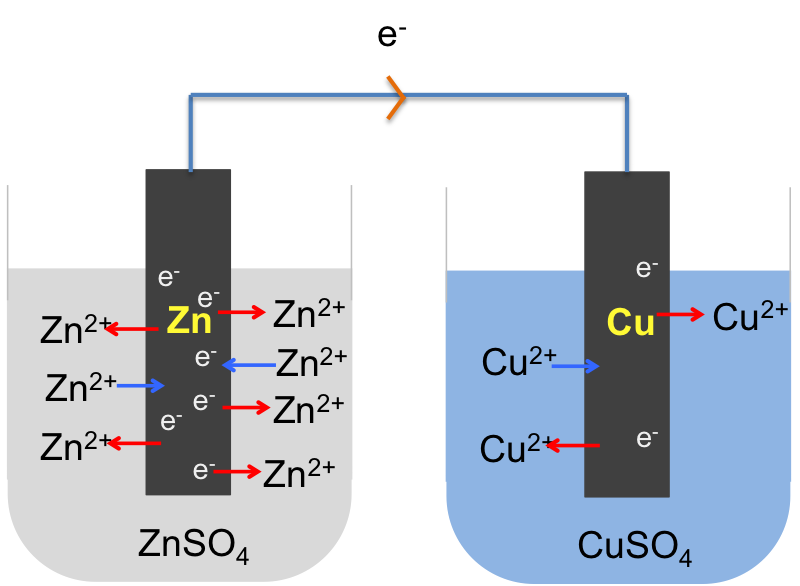
If we connect the zinc and copper electrodes with a wire, the electrons are no longer contained in the respective electrodes. They flow from a region of higher electrode potential (higher energy) to a region of lower electrode potential (lower energy), i.e. from the zinc electrode to the copper electrode (see diagram below). This difference in electrode potentials is called the potential difference between the two systems. The further apart the two metals are in the metal reactivity series, the greater the potential difference. We call the linked electrodes and their respective salt solutions, an electrochemical cell, and the individual metal/metal salt solution system, a half-cell.
The overall electrochemical reaction is:
next article: Electrochemical cell
