The pH scale is a range of numbers for measuring the extent of acidity or basicity of a substance.
As mentioned in the previous article, pH is defined as –log[H+] and is accurate over the range of 0 to 14, which is equivalent to concentrations of aqueous H+ of between 1 M to 10-14 M. We can use the values of 0 to 14 to construct a colour-coded pH scale as a convenient reference for the concentration of H+ in solutions.
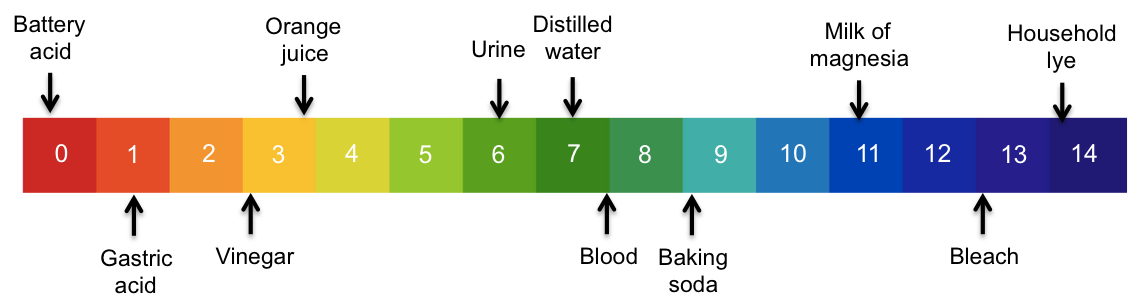
So, instead of saying that the concentration of H+ in a sample of urine is 10-6 mol dm-3, we simply say that the sample of urine has a pH of 6.

Question
What if the concentration of H+ in a solution is 2 M? Does it mean that the pH of the solution is -0.3?
Answer
We often use a pH glass electrode to measure the pH of an aqueous solution. While it is mathematically possible to calculate a pH of -0.3 for a 2 M HCl solution, the practical measurement of pH becomes less accurate at very high concentrations (typically above 1 M). This is because the activity of ions in concentrated solutions deviates significantly from their ideal concentrations due to interionic interactions, making it challenging for the electrode to accurately reflect the true values. Furthermore, acid molecules may be absorbed by a layer of gel, which is coated on the glass bulb (see below diagram), lowering the activity of H+ at the electrode, making it harder to accurately measure the real concentration of H+ in the solution. On the other hand, a solution with pH < 10-14 M has so low a concentration of H+ that other cations (such Na+) present in the solution may be detected by the electrode instead, giving an inaccurate reading. This is why the pH scale is usually quoted between 0 to 14. Fortunately, many common solutions like those stated in the diagram above have pH values within the measurable range.

