Beyond the stoichiometric point of titration, the pH of the solution experiences significant shifts, often leading to rapid changes that highlight the excess of titrant and the resultant dominance of the strong base or acid in the solution.
To characterised this region, we make the following assumption:
-
- [OH–] from water is negligible, i.e. pH of the solution is solely determined by [OH–]ex from the excess base added after the stoichiometric point, as the dissociation of water is again suppressed at this stage.
Even though the auto-dissociation of water is suppressed, water is still equilibrating between its molecular form and its ionic components. Applying the above assumption, the equilibrium constant of water is:
Taking the logarithm on both sides of the above equation and applying the definitions of pH and pKw,
Eq5 is the general equation to calculate the pH of a strong base to weak acid titration beyond its stoichiometric point. The same logic applies when determining the equation for the pH of a strong acid to weak base titration beyond the stoichiometric point.
The diagram below shows the superimposition of eq5 (purple curve) over the complete pH titration curve (blue curve), which disregards the above assumption, for the titration of 10 cm3 of 0.200 M of CH3COOH (Ka = 1.75 x 10-5) with 0.100 M of NaOH.
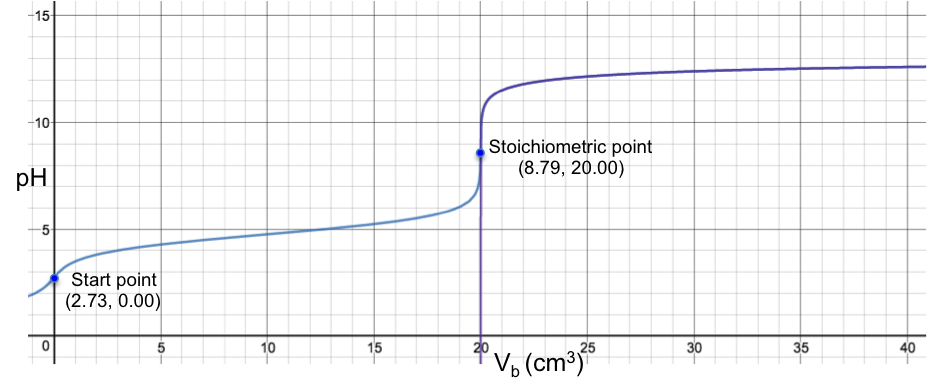
Even though the two curves appear to fit perfectly, they do not actually coalesce and are still two separate curves (discernible when the axes of the plot are scaled to a very high resolution). However, for practical purposes, eq5 is a very good approximation of a pH curve for the region beyond the stoichiometric point of a strong acid to weak base titration.