The electrochemical cell below contains two electrolytes, aqueous zinc sulphate and aqueous copper sulphate, that are separated by a semi-permeable membrane, which in this case, is an anion-exchange membrane (AEM). The AEM, made with positively charged polymers, allows only anions in the electrolyte to pass through it, with the cations remaining in their respective compartments. It serves to maintain electrical neutrality in both compartments and to complete the electrochemical circuit.
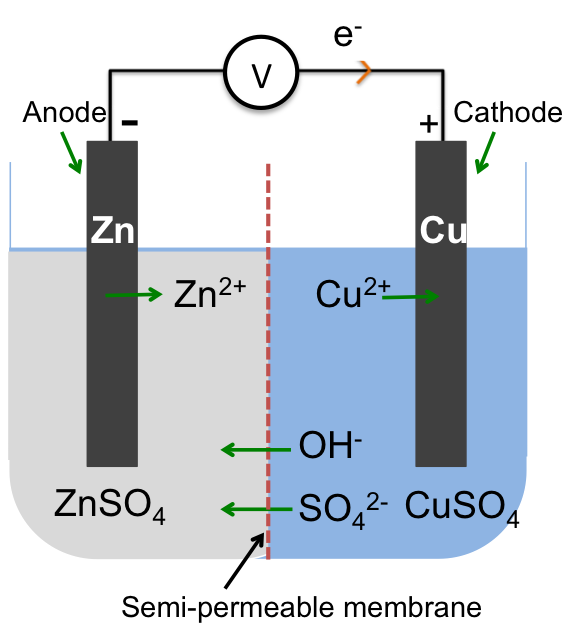
To determine the reactions in the cell, we follow the steps outline in the previous article, which result in the following overall redox reaction equation:
Zn (s) + Cu2+ (aq) → Zn2+ (aq) + Cu (s)
As the reaction progresses, the blue copper sulphate solution decolourises when Cu2+ ions are reduced to Cu.
Another way to construct an electrochemical cell is shown in the diagram below. The electrolytes are housed in separate vessels that are linked by a salt bridge, which is a gel-filled glass tube containing a suitable electrolyte. The preferred electrolyte in the salt bridge is one that does not react with the electrolytes in the vessels and with the electrodes.
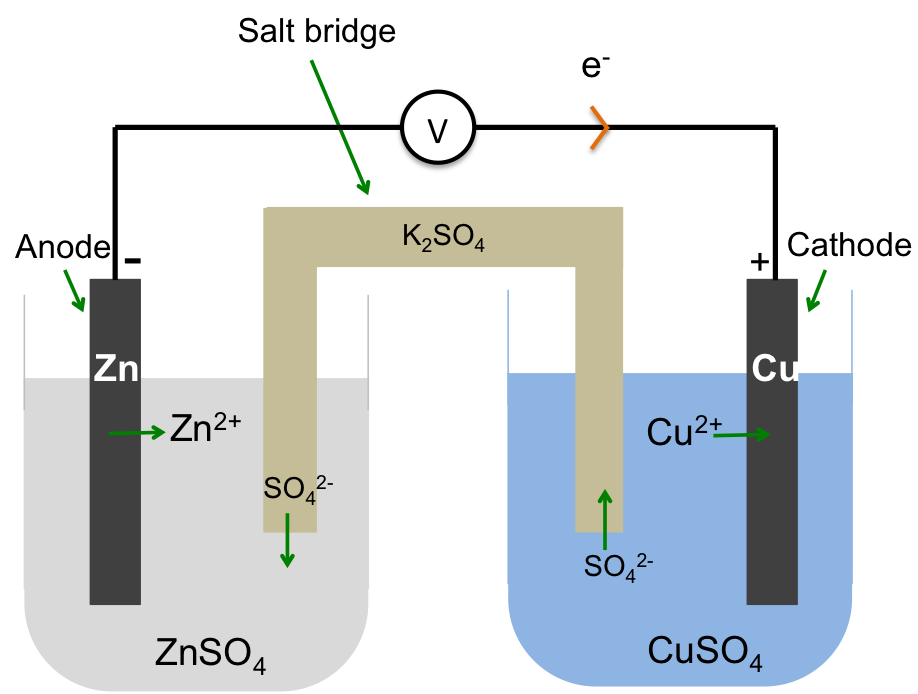
Like the semi-permeable membrane in the earlier setup, the salt bridge maintains electrical neutrality in both vessels and completes the electrochemical circuit by allowing the flow of anions from one vessel to the other.