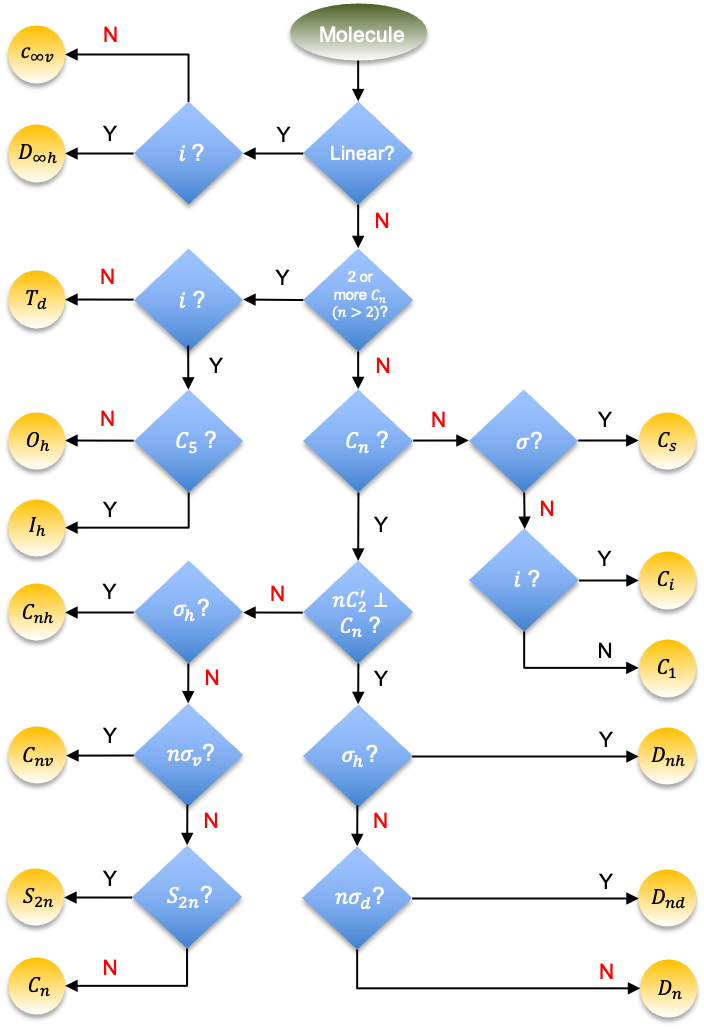
Molecular point groups are point groups to which molecules are assigned according to their inherent symmetries. They are used for analysing and predicting chemical properties of the assigned molecules.

The laborious way to identify the point group of a molecule is to visually determine all the symmetry operations associated with the molecule and compare them with the sets of symmetry operations of all point groups. A less arduous method involves finding key symmetry elements of a molecule and matching them sequentially to the characteristic symmetry operations of a point group. For example, cyclohexane in its twisted boat form (see diagram above) has two axes perpendicular to the main
axis, while the dihedral groups
and
all have in common, the elements
and
. Furthermore, the molecule does not have any mirror plane and hence belongs to the point group
. Similarly, a molecule with at least one
axis belongs to either
or
, while a molecule with two or more
, where
, belongs to one of the cubic or icosahedral point groups. We can summarise such an identification logic in the following flow chart: