To analyse reactions in the PbBr2 electrolytic cell (see previous article), we follow these steps:
Step 1: Determine the direction of flow of electrons
The flow of electrons in an electrolytic cell is determined by the way the battery (direct current source) is connected to the circuit. The battery is represented by two parallel lines with the longer line symbolising the positive terminal and the shorter one, the negative terminal.
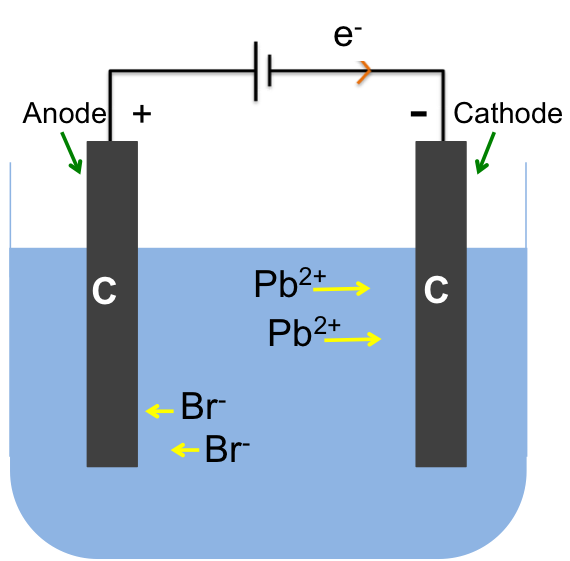
For this example, the positive terminal of the battery is connected to the left electrode, while the negative terminal to the right electrode. Electrons flow from the negative terminal to the right electrode making it negatively charged. The negatively charged electrode attracts cations in the electrolyte towards its surface, where they undergo reduction. Hence, the electrode that is connected to the negative terminal of the battery is the cathode. Using the same logic, oxidation takes place at the left electrode, which is the anode.
Step 2: Find out the reduction reaction at the cathode by
-
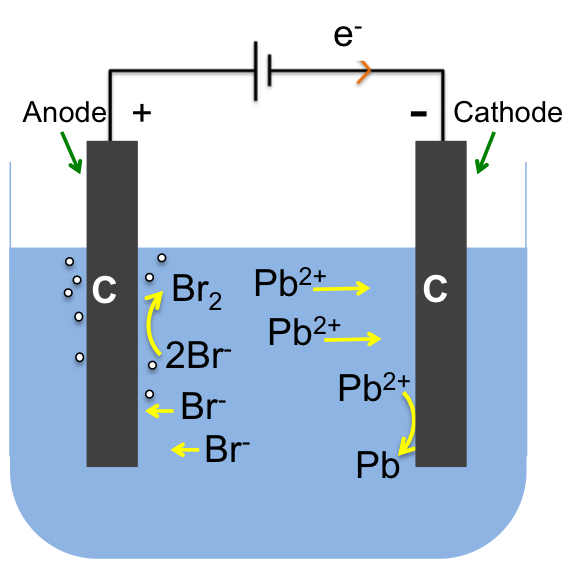
- Identifying the reducible species (usually positive ions), which in this case is Pb2+. There is only one reducible species, as the electrolyte is molten PbBr2 and the electrodes are inert.
- Distinguishing the species that is most readily reduced if there is more than one competing species by:
- comparing the species on the electrochemical series: N.A.
- comparing the concentrations of the species if their positions in electrochemical series are relatively near: N.A.
- Writing the ionic half-reaction equation for the reduction:
Pb2+ (l) + 2e– → Pb (l)
Step 3: Find out the oxidation reaction at the anode by
-
- Identifying the oxidisable species (usually negative ions): Br–
- Distinguishing the species that is most readily oxidised if there is more than one competing species by:
- comparing the species on the electrochemical series: N.A.
- comparing the concentrations of the species if their positions in electrochemical series are relatively near: N.A.
- Writing the ionic half-reaction equation for the reduction:
2Br– (l) → Br2 (g) + 2e–
Combining the two half reaction equations, the overall redox reaction in the electrolytic cell is:
Pb2+ (l) + 2Br– (l) → Pb (l) + Br2 (g)
Note that the electrolytic circuit is complete by the flow of electrons from the anode to the positive terminal of the battery via the wire and a net flux of negative ions towards the anode and positive ions towards the cathode in the electrolyte.

Question
What are the products at the anode and cathode for the following electrolytic cells?
| Cell | Electrode pair |
Electrolyte |
|
1 |
C/C |
Concentrated NaCl |
|
2 |
Pt/Pt |
Acidified H2O |
|
3 |
C/C |
Dilute CuSO4 |
|
4 |
Cu/Cu |
Dilute CuSO4 |
Answer
|
Cell |
Species at anode |
Products at anode |
|
1 |
Cl–, H2O |
Cl2, since [Cl–] >> [H2O] |
|
2 |
H2O |
O2 |
|
3 |
H2O, SO42– |
O2, since H2O is much higher in the electrochemical series than SO42- |
|
4 |
Cu, H2O, SO42– |
Cu2+, since Cu is higher in the electrochemical series than H2O & SO42-, and [Cu] >> [SO42-] & [H2O] |
H2O is always assumed to be the species instead of OH– in non-hydroxide aqueous solutions, as the concentration of OH– from H2O is very small. Similarly, H2O is assumed to be the species instead of H+ in non-acidic aqueous solutions, as the concentration of H+ from H2O is very small.
|
Cell |
Species at cathode |
Products at cathode |
|
1 |
Na+, H2O |
H2, since H2O is much lower in the electrochemical series than Na+ |
|
2 |
H+, H2O |
Both species are reduced to H2, with H+ undergoing reduction first, as it is much lower in the electrochemical series than H2O. H2O will be reduced when [H+] falls appreciably. |
|
3 |
Cu2+, H2O |
Cu, since Cu2+ is much lower in the electrochemical series than H2O |
|
4 |
Cu2+, H2O |
Cu, since Cu2+ is lower in the electrochemical series than H2O |
