Titration curves reveal important data of acid-base systems, such as the pH at the stoichiometric point, the pH at maximum buffer capacity, equilibrium constants, and more. Their shapes are governed by the equilibria of the acid, the base and water. In general, titrations are categorised into four groups:
-
- Strong acid versus strong base
- Weak acid versus strong base
- Strong acid versus weak base
- Weak acid versus weak base
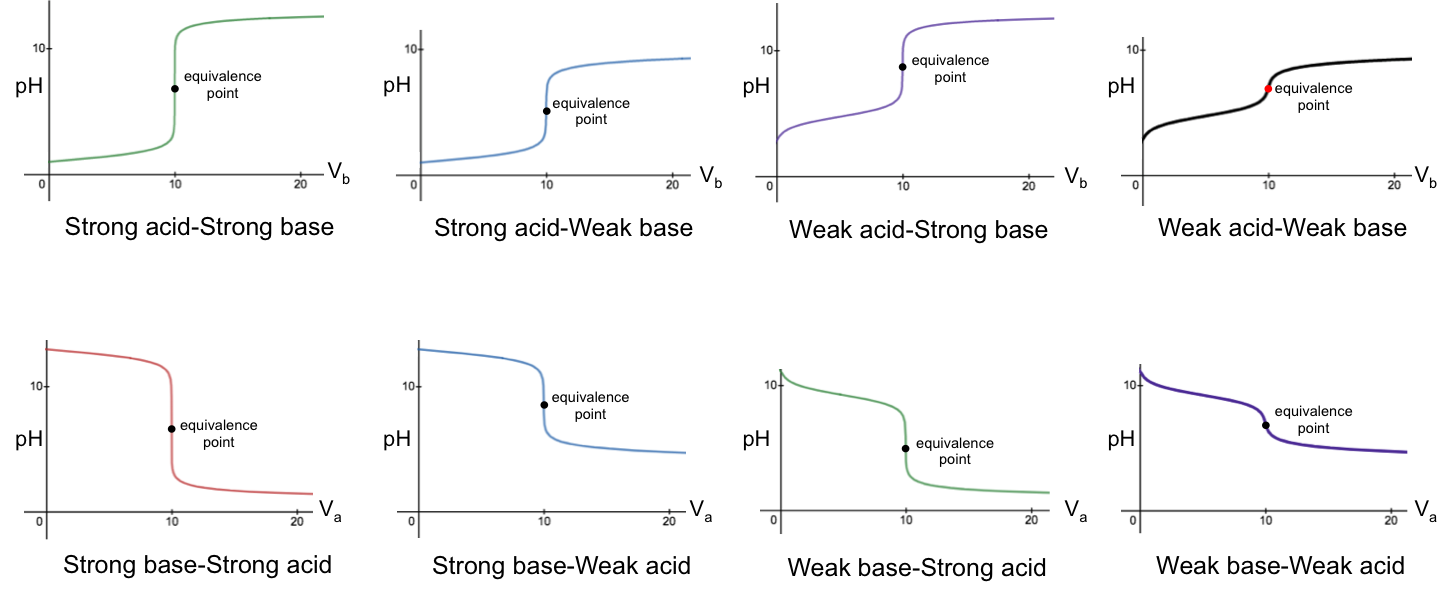
The pH-volume profile of each group can be expressed explicitly using a unique pH titration curve formula. Knowing the formulae for all categories therefore allows us to explain mathematically the shape of the curves, including the reasons for the sharp change of pH near the stoichiometric points. In the following derivation of the pH curve equations for the four categories of titration, we assume that the activity of every chemical species is equal to its concentration.