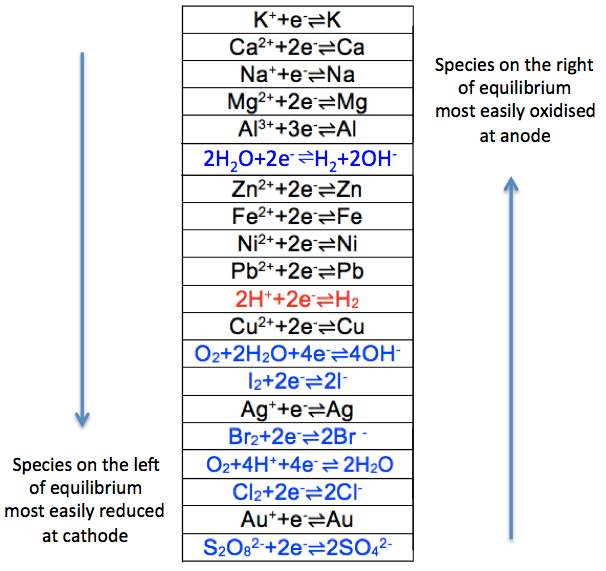
The electrochemical series is a tabulation of the half-cell standard electrode potentials of various chemical species.
Just as the metal reactivity series compares the reactivity of metals, the electrochemical series lists the reactivity of metals and ions in an electrochemical cell. A summary of the electrochemical series is as follows:-
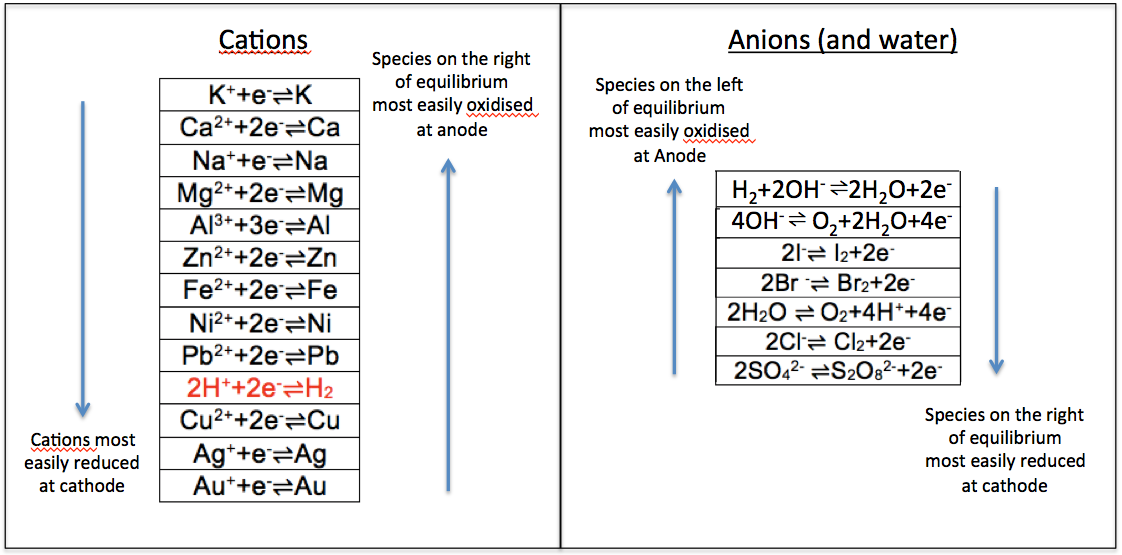
Since potassium is at the top of the cation series, its elemental form K is the most reactive and, therefore, most easily oxidised to its ionic form at the anode, compared to the other elements in the series. This implies that its ionic form K+ is least easily reduced at the cathode compared to the other ions in the series. The same logic applies to anions. Note that the oxidation of the sulphate (and nitrate) ion at the anode is relatively thermodynamically infeasible, as the anion is quite stable. The two series can be combined into one, in the form of reduction potentials (i.e. oxidised-to-reduced from left to right) as follows: